Hydrogen sulfide is a gaseous compound with the chemical formula H2S. It possesses significant corrosive, flammable, and toxic properties. The odor emitted is highly offensive and bears a strong resemblance to the scent of decomposed eggs. Known as a colorless chalcogen hydride, hydrogen sulfide is a highly recognizable chemical compound that can be readily identified.

Hydrogen sulfide is predominantly generated as a result of the microbial decomposition of organic substances in anaerobic conditions. This phenomenon primarily occurs within wetland ecosystems and underground sewage systems and is commonly referred to as anaerobic digestion. The primary agents responsible for this process are microorganisms that reduce sulfates. Hydrogen sulfide is also present in volcanic emissions, unrefined petroleum, natural gas, and specific sources of well water or geothermal springs. It is noteworthy that the human body also synthesizes minute quantities of hydrogen sulfide, which serves as a signaling molecule.
| Properties of Hydrogen sulfide H2S | |
| Chemical formula | H2S |
| Molar mass | 34.08 g·mol-1 |
| Appearance | Vapor pressure |
| Odor | Pungent, like that of rotten eggs |
| Density | 1.363 g dm-3 |
| Melting point | −82 °C (−116 °F; 191 K) |
| Boiling point | −60 °C (−76 °F; 213 K) |
| Solubility in water | 4 g dm-3 (at 20 °C) |
| Vapor pressure | 1740 kPa (at 21 °C) |
| Acidity (pKa) | 7.0 |
| Conjugate acid | Sulfonium |
| Conjugate base | Bisulfide |
| Magnetic susceptibility (χ) | −25.5·10-6 cm-3/mol |
Interesting Science Videos
Preparation of Hydrogen Sulfide (H2S) in Kipp’s Apparatus
- Kipp’s apparatus is a specially developed device designed to procure intermittently products of commonly utilized gases, including hydrogen, carbon dioxide, as well as hydrogen sulfide.
- Hydrogen sulfide gas is important as a reagent and is extensively used in qualitative analysis.
- The constant production of hydrogen sulfide (H2S) gas inside a laboratory setting not just results in the waste of gas resources but also poses a significant health hazard as it is a toxic gas known to induce symptoms such as headaches.
- Kipp’s apparatus effectively mitigates these issues, and its utility in generating H2S gas at regular intervals has been demonstrated. To clarify, the generation of hydrogen sulfide (H2S) gas is regulated through the utilization of Kipp’s apparatus.

- When tap A is opened, dilute sulfuric acid flows into bulb B and reacts with the iron (II) sulfide, resulting in the production of hydrogen sulfide. This hydrogen sulfide is then delivered through tap A.
- A is turned off when the gas is no longer required.
- Gas continues to be generated, causing an increase in pressure within B.
- As a result, the acid is expelled from B and travels upward into C.
- The generation of hydrogen sulfide ceases as the acid and iron (II) sulfide are no longer in contact. The apparatus will remain inactive until tap A is reopened to obtain gas.
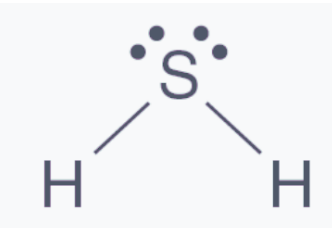
Hydrogen Sulfide Structure
Hydrogen sulfide has a structure that resembles water. Hydrogen sulfide is made up of two hydrogen atoms and one sulfur atom, according to the Lewis structure. The main component is sulfur, which is composed of two lone hydrogen atoms joined together by a single bond.
Physical Properties of Hydrogen Sulfide
- Hydrogen sulfide is a colorless gas that has an unpleasant odor similar to that of rotten eggs.
- Hydrogen sulfide exhibits a notable level of solubility in water. When dissolved, the resulting solution retains the characteristic odor of the gas, with a ratio of 3 volumes of gas per 1 volume of water.
- Hydrogen sulfide gas is inherently toxic. Inhaling small amounts of it can cause headaches, while prolonged consumption may lead to mortality.
- Hydrogen sulfide is denser than air and has a vapor density of 17.
- Hydrogen sulfide can be readily liquefied at a temperature of -61 °C and solidified at an even lower temperature of -85 °C.
Chemical Properties of Hydrogen Sulfide
Combustibility
The H2S possesses the ability to undergo combustion, yet it does not act as a facilitator or enhancer of the combustion process. The combustion process results in the emission of a blue-colored flame, leading to the production of sulfur and water as byproducts. Sulfur dioxide is formed in an environment with a high level of oxygen.
Thermal Dissociation
Upon reaching a temperature exceeding 400 degrees Celsius, the H2S undergoes dissociation, resulting in the separation of sulfur and hydrogen. The dissociation process reaches full completion when the temperature is raised to 1700 degrees Celsius.
Acidic Nature (as a Weak Acid)
Hydrogen sulfide functions as a relatively low-strength dibasic acid. When Sodium Hydroxide (NaOH) reacts, it produces two salts, namely normal sodium sulfate (Na2S).
2 NaOH (aq) + H2S (g) → Na2S (aq) + 2 H2O (l)
or, When there is an abundance of hydrogen sulfide, the resulting compound is the acid salt known as sodium hydrogen sulfide. NaHS.
The chemical equation representing the reaction between sodium hydroxide (NaOH) in aqueous solution and hydrogen sulfide (H2S) in gaseous form is as follows:
NaOH (aq) + H2S (g) → NaHS (aq) + H2O (l)
Potassium hydroxide exhibits similar reactivity.
The potential reactions between hydrogen sulfide and sodium hydroxide solution include:
2 NAOH(aq) + H2S (g) → Na2S (aq) + 2 H2O (l)
2 NaOH (aq) + 2 H2S (g) → 2 NaHS(aq) + 2 H2O (l)
The equation indicates that the quantity of hydrogen sulfide required to convert a specific mass of sodium hydrogen sulfide, NaHS, is twice as much as the quantity needed to convert it to sodium sulfide, NaS.
In practical terms, it is challenging to precisely ascertain the optimal point at which to carry out the preparation process, wherein half of the sodium hydroxide is converted into sodium hydrogen sulfide through saturation with hydrogen sulfide.
After that, adding the remaining half of the sodium hydroxide allows the formation of the normal salt from the acid salt.
NaOH (aq) + H2S (g) → NaHS (aq) + H2S (l)
NaHS (aq) + NaOH (aq) → Na2S (aq) + H2O (l)
The aqueous solution of hydrogen sulfide (H2S) exhibits acidic properties and is commonly referred to as hydrosulfuric acid. Due to its relatively lower acidity compared to carbonic acid, hydrogen sulfide (H2S) does not undergo carbonate decomposition. Nevertheless, it reacts with metal oxides to produce corresponding sulfides.
CaO + H2S → CaS + H2O
ZnO + H2S → ZnS + H2O
Reducing Properties
The strong reducing properties of hydrogen sulfide can be attributed to its high susceptibility to oxidation. Like other reducing agents, it operates by supplying a source of electrons. The customary consequence of a chemical reaction is the formation of a solid sulfur precipitate, which arises from the observed modifications.
H2S ⇌ 2 H+ + S2-
S2- → S + 2e–
The oxidizing agent accepts the electrons from the hydrogen sulfide during the reaction.
(i) It Reduces Ferric Salts To Ferrous
2 FeCl3 + H2S → 2 FeCl2 + 2 HCl + S
Fe2(SO4)3 + H2S → 2 FeSO4 + H2SO4 + S
(ii) It Reduces Chlorine, Bromine, and Iodine to Respective Haloacids
Cl2 + H2S → 2 HCl + S
Br2 + H2S → 2 HBr + S
I2 + H2S→ 2 HI + S
(iii) It Reduces Hydrogen Peroxide to Water
H2O2 → H2O + [O]
H2S + [O] → H2O + S
H2O2 + H2S → 2 H2O + S
(iv) It Reduces Sulfur dioxide to Sulfur
SO2 + 2 H2S → 2 H2O + 3 S
(v) It Decolorizes and Forms Salts
The decolorization of acidified KMnO4 solution results in the formation of manganous salt, while the acidified potassium dichromate solution undergoes a color change to form chromic salt, which appears green.
(a) 2 KMnO4 + 3 H2SO4 → K2SO4 + 2 MnSO4 + 3 H2O + 5[O]
H2S + [O] → H2O + S]5
2 KMnO4 + 3 H2SO4 + 5 H2S → K2SO4 + 2 MnSO4 + 8 H2O + 5S
Violet (purple) colorless
(b) K2Cr2O7 + 4 H2SO4 → K2SO4 + Cr2(SO4)3 + 4 H2O + 3[O]
H2S + [O] → H2O + S]3
K2Cr2O7 + 4 H2SO4 + H2S → K2SO4 + Cr2(SO4)3 + 7 H2O + 3S
(Orange-Red) (Green)
(vi) It Reduces Nitric and Sulfuric Acid
The reduction of nitric acid resulted in the formation of nitrogen peroxide, while the reduction of sulfuric acid yielded sulfur dioxide.
2 HNO3 + H2S → 2 H2O + 2 NO2 + S
H2SO4 + H2S → 2 H2O + SO2 + S
Analytical Reagent
H2S is a widely used analytical reagent for detecting and separating different metal ions. When hydrogen sulfide (H2S) is introduced into salt solutions in an acidic or ammoniacal environment, it results in the formation of metal sulfides. These metal sulfides display distinct colors that are characteristic of each metal.
CuSO4 + H2S + H+→ CuS ↓ Black + H2SO4
ZnSO4 + H2S + OH– → ZnS ↓+ H2SO4
Applications of Hydrogen Sulfide
Hydrogen sulfide serves a crucial role as a reagent of significance in the context of qualitative analysis.
The precipitation of metal sulfides from a salt solution using H2S provides a technique for separating and identifying basic radicals in qualitative analysis. This is due to the distinct colors exhibited by these sulfides and their precipitation in various media.
(i) In Acidic Solution
When hydrogen sulfide (H2S) is introduced into an acidic salt solution containing hydrochloric acid (HCl), the sulfides of metallic radicals from group II in quantitative analysis are precipitated. These precipitates exhibit distinct colors, which are as follows:
(Acidic Medium)
CuSO4 + H2S → CuS ↓ (Black ppt.) + H2SO4
Colors of Precipitate:
HgS, CuS, and PbS → Black
Bi2S3 → Brown
As2S3, and CdS → Yellow
Sb2S3 → Orange
SnS2 → Dirty Yellow
Sns → Chocolate Brown
(ii) In Alkaline Solution
Sulfides with a relatively high solubility are exclusively precipitated from an ammoniacal solution (basic solution) using H2S. Zinc, manganese, cobalt, and nickel are precipitated in a similar manner within the III B group of qualitative analysis. In this experimental procedure, a salt solution is subjected to an excessive quantity of NH4Cl and subsequently rendered alkaline through the introduction of NH4OH. The process involves the introduction of H2S gas into the system, resulting in the precipitation of metallic sulfides. The sulfides possess the following coloration:
(Basic medium)
ZnSO4 + H2S → ZnS ↓ (white ppt.) + H2SO4
(Basic medium)
MnSO4 + H2S → MnS ↓ (Flesh color ppt.) + H2SO4
Hydrogen Sulfide Toxicity
- The eyes, nose, and throat may get irritated when exposed to low quantities of hydrogen sulfide. Some asthmatics may experience breathing difficulties as a result of it. Low levels of hydrogen sulfide might result in fatigue, memory loss, headaches, and balance issues.
- Short-term exposures to hydrogen sulfide at levels above 1000 ppm can result in unconsciousness. Most often, there are no further consequences and the victim seems to regain consciousness.
- However, some people may experience long-lasting or permanent symptoms, such as headaches, inability to focus, poor memory, and impaired motor skills.
References
- https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen-Sulfide
- https://byjus.com/jee/hydrogen-sulphide/
- https://www.osha.gov/hydrogen-sulfide
- https://www.sciencedirect.com/topics/engineering/hydrogen-sulfide
- Lissauer, Jack J.; de Pater, Imke (2019). Fundamental Planetary Sciences: physics, chemistry, and habitability. New York, NY, USA: Cambridge University Press. pp. 149–152. ISBN 9781108411981.
- Committee on Medical and Biological Effects of Environmental Pollutants (1979). Hydrogen Sulfide. Baltimore: University Park Press. ISBN 978-0-8391-0127-7.
- https://hnhu.org/health-topic/what-is-hydrogen-sulphide-h2s/
