Identification of gases includes the observation of their physical and chemical properties, conducting experiments with them, or using specialized instruments like gas chromatography or mass spectrometry. To identify gases, you can observe their density, color, odor, and how they react with other chemicals.
There are a variety of applications for gases, including carbon dioxide, hydrogen, and oxygen, all of which may be found in ample supply in our natural environment. Scientists rely on the features that are unique to certain gases to identify them. A quality that may be observed is referred to as a characteristic property.
Interesting Science Videos
Common Gases And Identification of Gases
Here we will learn the techniques to identify the common gases in the atmosphere.
Hydrogen Gas (H2)
When burned in the presence of air, the odorless, colorless gas known as hydrogen makes a squeaky pop sound. Hydrogen has no color or odor. When burned, H2 emits a light blue flame, can put out fires started by burning splinters, and does not react to a litmus test. It produces water through a reaction that takes place very quickly with oxygen.
Carbon-dioxide Gas (CO2)
Carbon dioxide can be detected in two ways: visually and chemically. It puts out a burning splinter. Any gas lacking oxygen in any form will give this result, making this a useless test. This leads to the misidentification of mostly hydrogen gas.
Visual Identification: Carbon dioxide can be tested by bubbling it through lime water. Lime water will turn milky if the test is successful. Calcium carbonate crystals form, which accounts for the milky appearance. It precipitates out of water as a milky-white substance because it is insoluble.
Chemical Identification: It turns moist blue litmus red and often evolves with quick foaming.

[Image source: BBC]
Oxygen Gas (O2)
Oxygen is a colorless gas with no distinctive odor. It is a combustible gas. Therefore, it will reignite a splint that is glowing. O2 also changes the alkaline pyrogallol solution (which is colorless) into a brown color. It is also neutral to the litmus test.
Chlorine
Chlorine is an acidic gas that also serves as a bleach. When exposed to chlorine, damp litmus paper undergoes a process of bleaching, resulting in a white coloration.
When damp blue litmus paper is utilized, it undergoes a color change and turns from blue to red, and eventually to white.
Ammonia
The ammonia test is a method used to detect the presence of ammonia in a given sample. Ammonia possesses a distinctive odor. The olfactory sensation of ammonia is often characterized as a suffocating odor.
Litmus paper is capable of detecting the presence of ammonia. Litmus paper is utilized in the process of detecting ammonia. The litmus paper must be moistened.
The application of litmus paper results in a blue color change. The presence of ammonia gas can be detected by observing a change in the color of litmus paper to blue.
The identification of ammonia can be achieved through the utilization of concentrated hydrochloric acid. One possible method of identification involves exposing the substance to hydrogen chloride gas, which can be generated by using concentrated hydrochloric acid. The reaction will result in the production of a white smoke composed of ammonium chloride.
Nitrogen dioxide (NO2)
This exhibits a reddish-brown hue and possesses an odor that is known to cause irritation. When an acidified solution of potassium iodide is mixed with a solution of starch, the resulting solution will appear blue in color.
The moist blue litmus paper change color and turns red.
Hydrogen Sulfide Gas (H2S)
It is gas that smells like rotten eggs but has no color. It causes moist lead (II) ethanoate (acetate paper) to turn a dark black color. It turns moist blue litmus into the red.
Sulfur Dioxide (SO2)
The gas has no color and has a strong, unpleasant odor. It puts out a burning splinter. Acidified potassium dichromate (K2Cr2O7) solution changes color from orange to transparent green.
The solution of acidified potassium permanganate (KMnO4) changes from pink to clear and colorless.
Iodine (I2)
Iodine is a chemical element with the symbol I2.
The gas is violet in color and has no smell. When exposed to starch paper, it causes the paper to turn blue. Additionally, a layer of violet sublimate is created on the sides of the tube.
Flame Test
- Testing for hydrogen and testing for oxygen, both of these tests are flame tests.
- When a lit splint comes into contact with hydrogen gas (H2), it produces a distinct sound known as a ‘squeaky pop’.
- When a glowing splint is exposed to oxygen gas (O2), it starts burning again.
- Other sources suggest that a flame test can be used to detect carbon dioxide (CO2). When you put a burning splint into a test tube that is filled with CO2, the flame will be extinguished. The outcome is identical when using different gases. If you put a lit splint in a test tube filled with nitrogen gas, it will be extinguished.
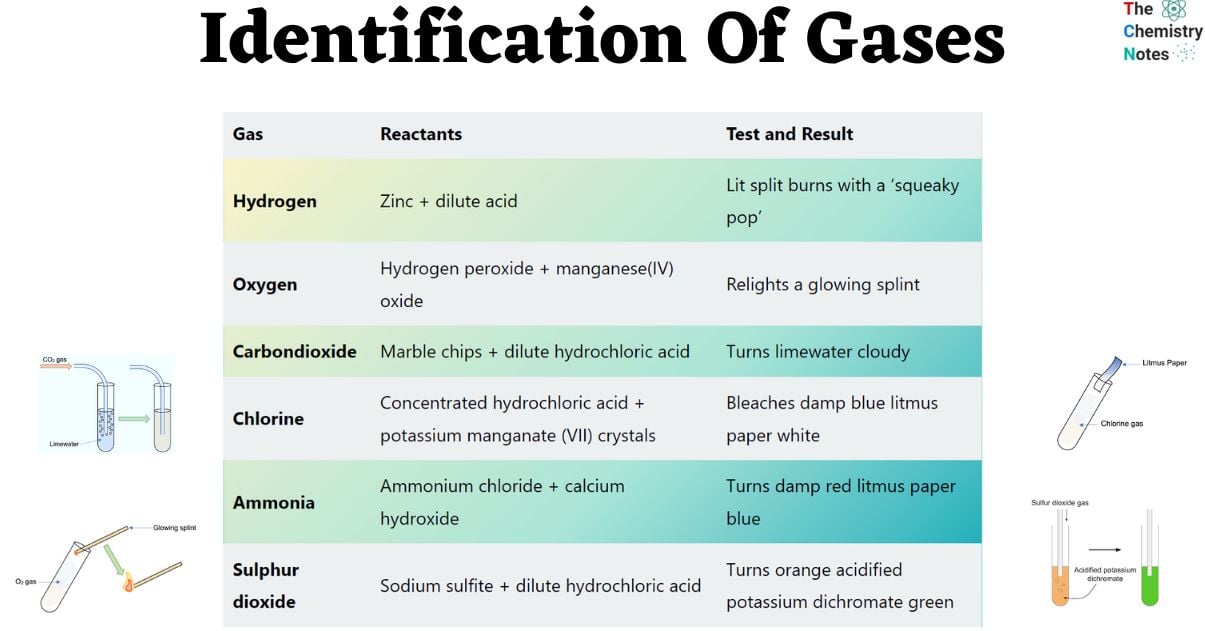
| Gas | Reactants | Test and Result |
| Hydrogen | Zinc + dilute acid | Lit split burns with a ‘squeaky pop’ |
| Oxygen | Hydrogen peroxide + manganese(IV) oxide | Relights a glowing splint |
| Carbondioxide | Marble chips + dilute hydrochloric acid | Turns limewater cloudy |
| Chlorine | Concentrated hydrochloric acid + potassium manganate (VII) crystals | Bleaches damp blue litmus paper white |
| Ammonia | Ammonium chloride + calcium hydroxide | Turns damp red litmus paper blue |
| Sulphur dioxide | Sodium sulfite + dilute hydrochloric acid | Turns orange acidified potassium dichromate green |
Frequently Asked Questions (FAQ)
What are Common Gases?
Common gases are gases that are commonly found in daily life and scientific experiments. Air, nitrogen, oxygen, carbon dioxide, and hydrogen are all common gases.
What are the methods for identifying between oxygen and carbon dioxide?
The burning splinter test can be used to identify oxygen. If the splint catches fire again when exposed to the gas, it means the gas is oxygen. To identify carbon dioxide, bubble it through lime water. When carbon dioxide is added to lime water, it creates calcium hydroxide which appears milky white.
Which gas produces thick white fumes when mixed with HCl?
When a rod that has been dipped in HCI is placed into a jar that contains ammonia gas, you will see thick white fumes of ammonia chloride.
Which gases can be collected by using the upward delivery method?
Gases that are lighter than air are collected by upward delivery. The gas jar is turned upside down so that lighter gases can be collected at the top, and the air inside the jar is pushed out. This is also called the Downward displacement of air. This process gathers gases like ammonia and hydrogen.
Video on Identification of Gases
References
- https://www.bbc.co.uk/bitesize/guides/zqqtrwx/revision/5
- https://studymind.co.uk/notes/identifying-common-gases/
- https://en.wikibooks.org/wiki/9-1_Chemistry/Identification_of_common_gases
- https://www.lbq.org/search/chemistry/chemical-analysis/identification-of-common-gases/identification-of-common-gases
- https://www.embibe.com/exams/identification-of-gases/
- https://www.halewoodacademy.co.uk/downloads/2020-21/curriculum/homework_knowledge_organisers/science/seperates/chemistry/aqa_gcse_chemistry_separate_science_u8_chemical_analysis_knowledge_organiser.pdf
- https://edu.rsc.org/experiments/generating-collecting-and-testing-gases/693.article
- https://study.com/academy/lesson/tests-for-identifying-common-gases.html
- https://www.studysmarter.co.uk/explanations/chemistry/chemical-analysis/testing-for-gases/

