Intermolecular forces refer to the attraction between atoms, molecules, and ions nearby. This differs from intramolecular forces, which refer to covalent bonding within molecules.
When two particles have an intermolecular interaction, the positive (+) charge on one attracts the negative (-) charge on the other. Strong intermolecular forces attract and bring atoms, molecules, and ions closer together. These are typically found in condensed states, such as liquids or solids.
Intermolecular forces are electrostatic in origin, meaning they result from the interaction of positively and negatively charged species. Intermolecular interactions, like covalent and ionic bonds, are a combination of attracting and repulsive forces.
Interesting Science Videos
What is intermolecular force?
Every molecule or atom attracts or repels other substances. This force is referred to as the intermolecular force. These forces are responsible for the cohesiveness of a substance. Intermolecular forces are classified into two types: attractive and repulsive forces.
Intermolecular attractive forces influence the properties of all states of matter. These qualities are determined by the strength of intermolecular forces and the types of intermolecular forces present, which vary in strength. This “strength” influences the “sticking together” of molecules, which in turn causes the change in physical attributes.
Intermolecular forces, as opposed to intramolecular forces, such as covalent bonds that keep atoms together in molecules and polyatomic ions, hold molecules together in liquids or solids. Intermolecular forces determine bulk qualities such as solid melting points and liquid boiling points. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attraction interactions that hold them together, generating vapor bubbles inside the liquid. Similarly, materials melt when molecules gain enough thermal energy to overcome the intermolecular interactions that hold them in place.
Intermolecular forces are significantly weaker than covalent bonds. At 100°C, it takes 927 kJ to overcome the intramolecular forces and break both O-H bonds in 1 mol of water, yet it only takes roughly 41 kJ to overcome the intermolecular attractions and convert 1 mol of liquid water to water vapor.
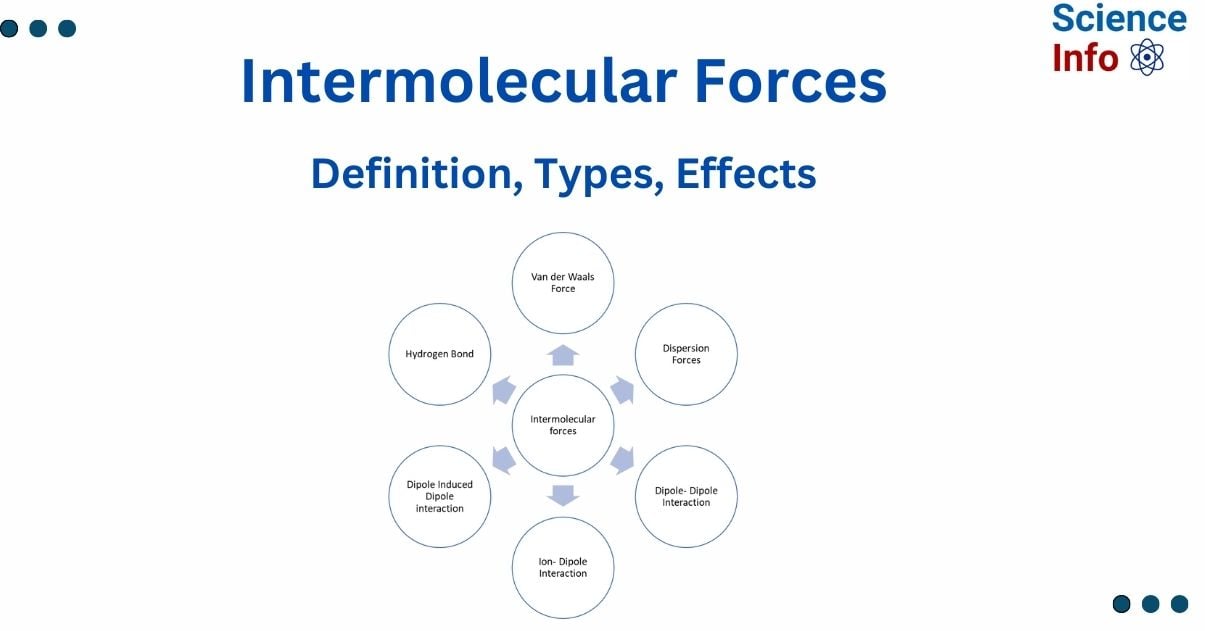
Types of Intermolecular Forces
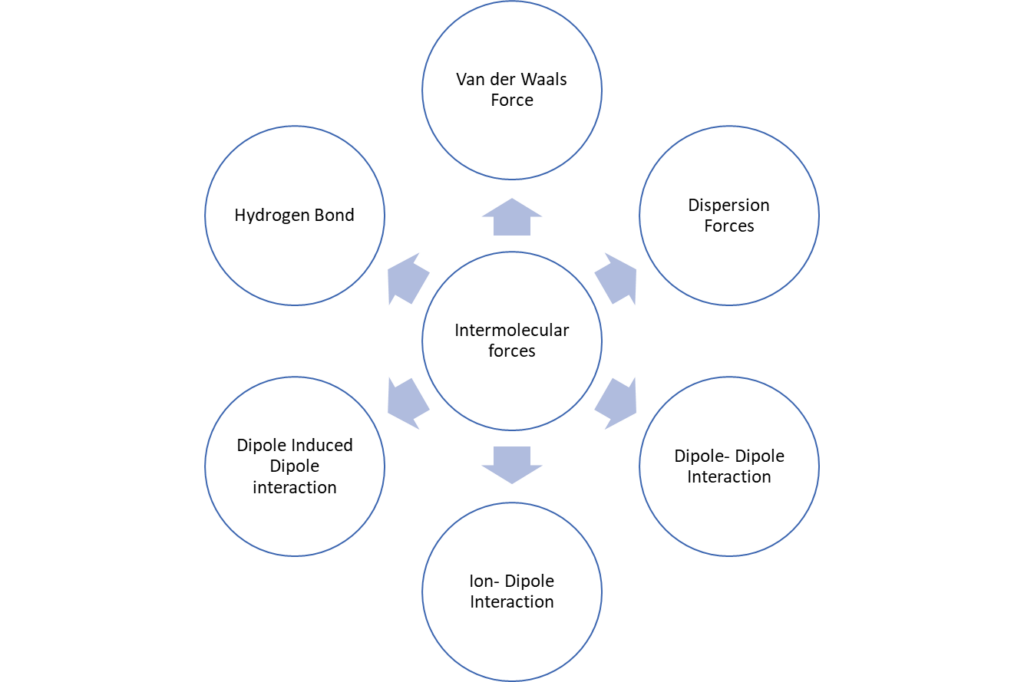
Hydrogen bond
A hydrogen bond is an attractive force between the hydrogen atom of one molecule bound and more electronegative atoms of the same molecule or other molecules. The hydrogen bond is weaker than a covalent bond, and thus, the bond length is longer. Fluorine, nitrogen, and oxygen are three electronegative elements that are commonly engaged in hydrogen bonds. Hydrogen bonds with sulfur, chlorine, and carbon have also been reported, but at a lower strength.
- The intramolecular hydrogen bond occurs between hydrogen atoms and electronegative atoms such as nitrogen, oxygen, and sulfur within the same molecule. An intramolecular hydrogen bond can only be formed when hydrogen and an electronegative atom are present nearby. Intramolecular hydrogen bonding in a molecule has a significant impact on the chemical structure and characteristics of the substance.
- Intermolecular hydrogen bonding is a type of hydrogen bonding that occurs between two separate molecules from the same or different substances. This sort of bonding can occur between any number of molecules, whether the same or different, as long as hydrogen and electronegative atoms exist.
Intermolecular hydrogen alters the characteristics of molecules, such as increasing surface tension and viscosity.
Van der Waals forces
Van der Waals forces refer to all attractive forces that exist between neutral atoms and molecules. The attraction is primarily due to electrostatic forces. However, there might be additional reasons for attraction between two or more elements of a substance. There are molecular attractions that do not necessarily require the presence of charges. These are known as the Van der Waals forces of attraction, and they exist in all molecules. They are a mix of Keesom forces, Debye forces, and London dispersion forces.
Dispersion force
One of the three van der Waals forces exists in all condensed phases, independent of the atoms or molecules that make up the substance. This attractive force is known as the London dispersion force The German-born American physicist Fritz London, first described it in 1928. This force is commonly referred to simply as the dispersion force. Dispersion forces between atoms in different compounds can attract them together. The forces, however, are quite mild and only become relevant when the molecules are extremely close.
Larger and heavier atoms and molecules have larger dispersion forces than smaller and lighter atoms. At normal temperature, F2 and Cl2 are gases (showing lesser attractive forces), while Br2 is liquid and I2 is solid.
The shapes of molecules influence the magnitudes of the dispersion forces between them. For example, the boiling temperatures of the isomers n-pentane, isopentane, and neopentane are 36, 27, and 9.5 °C, respectively. Even though these compounds have the identical chemical formula, C5H12, the variation in boiling points indicates that dispersion forces in the liquid phase differ, with n-pentane having the highest and neopentane having the lowest. The elongated structure of n-pentane increases the surface area accessible to contact between molecules, resulting in larger dispersion forces. Isopentane’s more compact form provides less surface area for intermolecular interaction, resulting in lesser dispersion forces.
London Dispersion forces (LDF) occur when electrons circulate across a molecule, causing temporary polarities to form. Molecules with larger molecular weights contain more electrons. Their electron clouds have a property known as polarizability, which increases their tendency to deform in response to external charges.
Molecules with larger molecular weights have higher LDF, which leads to higher melting, boiling, and vaporization points.
Dipole-Dipole force
Dipole-dipole forces exist between polar molecules with persistent dipole moments caused by uneven electron sharing. The uneven sharing of the molecule results in a partial positive charge (δ+) on one side and a partial negative charge (δ-) on the other. Molecules with opposite polarity tend to align, resulting in a condensed phase. Dipole-dipole attractions in substances lead to greater melting and boiling temperatures than LDF in nonpolar compounds.
As the dipole attraction occurs between two atoms that are close together, the force of attraction reduces as the distance between them increases. The dipole-dipole force changes with movement.
Ion-dipole interaction
Ion-dipole interactions are the electrostatic forces that attract an ion to a polar molecule. These
interactions are greater than dipole-dipole interactions because an ion has a significantly higher charge than a dipole. Ion-dipole interactions are stronger than hydrogen bonds. The ion-dipole interaction occurs when sodium chloride (NaCl) is dissolved in water and the sodium cation interacts with the partial negative charge on the oxygen atom of a water molecule.
Ion-induced dipole interaction
An ion positioned close to a non-polar molecule causes it to become polarised in this kind of interaction. When non-polar molecules become charged, they behave as induced dipoles. The interaction of an ion with an induced dipole is known as the ion-induced dipole interaction.
Dipole-induced dipole interactions
These interactions are comparable to ion-induced dipole interactions. The distinguishing feature is that non-polar molecules are changed into induced dipoles in the presence of a polar molecule nearby.
Instantaneous dipole-induced dipole interactions between nonpolar molecules can result in intermolecular attractions, much as they do in monatomic substances such as Xe. This impact increases with increasing atomic and molecular masses. For instance, Xe boils at -108.1°C, while He boils at -269°C.
Effects of intermolecular forces
- Stronger molecular forces bring molecules closer together, creating a condensed phase like liquid or solid. Molecules have to overcome intermolecular interactions to transition between solid, liquid, and gas phases.
- Stronger intermolecular forces demand more energy (heat) to transition from solid to liquid to gas.
- When the LDF increases with molecule weight, more energy is needed to melt (solid to liquid) and boil (liquid to gas) the halogen. At room temperature, halogens with stronger intermolecular forces are more condensed.
- In liquids, molecular attractions cause viscosity and surface tension.
References
- https://www.britannica.com/science/intermolecular-forces
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-The_Central_Science(Brown_et_al.)/11%3A_Liquids_and_Intermolecular_Forces/11.02%3A_Intermolecular_Forces.
- https://byjus.com/chemistry/different-types-of-intermolecular-forces/
- https://www.toppr.com/guides/chemistry/states-of-matter/intermolecular-forces/
- https://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/
