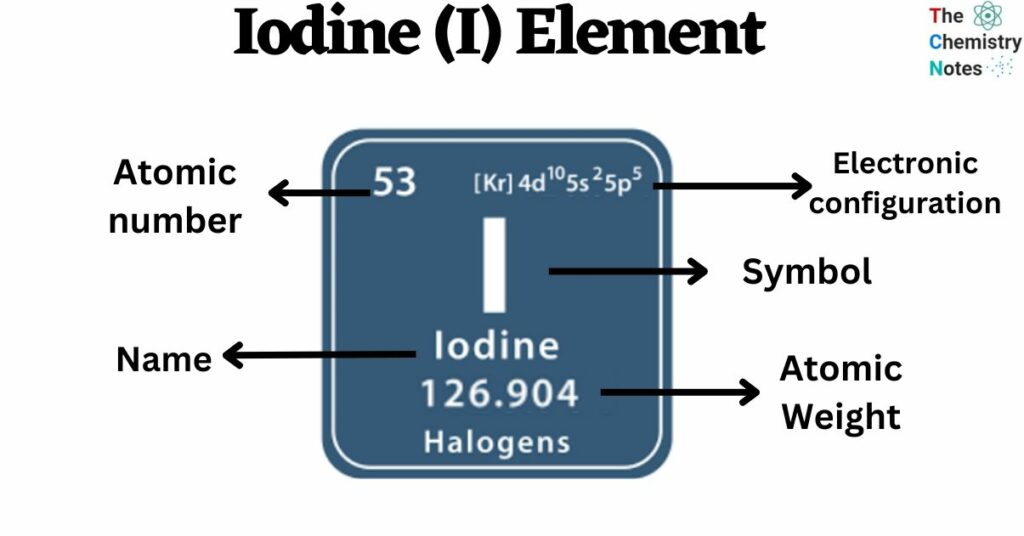
Iodine is a chemical element with the atomic number 53 and is represented by the symbol ‘I’ in the periodic table. It is a nonmetallic element and belongs to the p-block of group 17 of the periodic table. It is the heaviest of stable halogens.
Iodine ranks 61st in terms of abundance in Earth’s crust. With only 0.46 parts per million of the Earth’s crustal rocks containing iodine, it is the least prevalent of the stable halogens.
Interesting Science Videos
History of Iodine
- When French scientist Bernard Courtois applied sulfuric acid to seaweed ash in 1811 to extract sodium and potassium, he noted some purple vapors that condensed into crystals that exhibited a metallic shine. This led to the discovery of a new substance.
- Courtois shared a portion of the sample with two other researchers, Charles-Bernard Desormes and Nicole Clement (both French chemists) so that they could conduct additional tests on it.
- After the element was put on exhibit for the general public at the Imperial Institute in Paris in November of 1813, both Joseph Gay-Lussac and Humphry Davy independently determined that it should be added to the periodic table as a new element.
- When Humphry Davy reported his findings to the Royal Institution in London, he was incorrectly credited as the first person to make the discovery.
- The name of the element derives from the Greek word “iodes”, meaning violet. Because the iodine vapor has violet color it was given the name iodine.
Occurrence of Iodine
- In terms of abundance in the crust of the Earth, iodine comes in at number 61. Iodine is the least common of the stable halogens, occurring in the crustal rocks of the Earth.
- Its concentration in seawater is thought to be significantly lower, at only 0.0003 parts per million.
- There are just a few locations in the Earth’s crust where iodine appears to be concentrated. Oceans once surrounded these areas. Oceans disappeared over millions of years. The substances that had been dissolved in them are what they left behind. Later, earth tremors buried the dry chemicals that were left behind. As salt mines, they still exist today.
- Iodine minerals are uncommon, and the majority of deposits that are concentrated enough for profitable extraction are made up of iodate minerals. Lautarite, Ca(IO3)2, and dietzeite, 7Ca(IO3)2.8CaCrO4, are two examples.
- Iodine is also present in small amounts close to subterranean oil and gas sources. Additionally, some minerals and soils contain it.
Isotopes of Iodine
Iodine has a total of 37 isotopes with only 1 naturally occurring stable isotope: 127I.
Naturally Occurring Isotopes of Iodine
| Isotopes | Natural Abundance |
|---|---|
| 127I | 100 |
Elemental Properties of Iodine
| Electronic Configuration | [ Kr ] 4d10 5s2 5p5 |
| Atomic Number | 53 |
| Atomic Weight | 126.9045 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 17, 5, p-block |
| Density | 4.93 g.cm -3 at 20 °C |
| Ionic radius | 0.216 nm (-1) ; 0.05 nm (+7) |
| Van der Waals radius | 0.177 nm |
| Electron shells | 2, 8, 18, 18, 7 |
| Electrons | 53 |
| Protons | 53 |
| Neutrons in most abundant isotope | 74 |
Physical Properties of Iodine
- Iodine has an atomic number of 53 and is violet-black. It has a melting point of 113.7 °C (236.66 °F) and a boiling point of 184.3 °C (363.7 °F).
- Iodine has a solid phase density of 4.933 gm/cm3.
- Iodine is diamagnetic.
- Iodine has a strong odor.
- The elemental form of iodine is slightly soluble in water.
| Color/physical appearance | Lustrous, violet black |
| Melting point/freezing point | 386.85 K (113.7 °C, 236.66 °F) |
| Boiling point | 457.4 K (184.3 °C, 363.7 °F) |
| Density | 4.933 g cm-3 at 20° |
| Malleability | No |
| Ductility | No |
| Electronegativity | 2.66 (Pauling Scale) |

Chemical Properties of Iodine
- Iodine is non-flammable and toxic.
- Iodine does not combine with oxygen directly.
- Iodine is highly corrosive.
- It can evaporate when heated.
Chemical Reaction of Iodine
- The Reaction of Iodine With Air
Iodine, I2 does not react with oxygen, O2, or nitrogen, N2. However, iodine reacts with the second allotrope of ozone, O3, forming unstable yellow I4O9, the nature of which is perhaps I(IO3)3.
- The Reaction of Iodine With Water
When Iodine, I2, reacts with water it produces hypoiodite, OI–. The pH of the solution directly impacts the position of equilibrium.
I2 (l) + H2O (l) ⇌ OI– (aq) + 2H+ (aq) + I–(aq)
- The Reaction of Iodine With Halogens
Iodine reacts with fluorine in different temperatures
Iodine, I2, reacts with fluorine, F2, at room temperature to form the pentafluoride iodine (V) fluoride.
I2 (s) + 5 F2 (g) → 2 IF5 (l) [colourless]
When Iodine, I2, reacts with fluorine, F2, at 250 °C, the same reaction affords the heptafluoride iodine (VII) fluoride.
I2 (g) + 7 F2 (g) → 2 IF7 (g) [colourless]
When Iodine, I2, is reacted with fluorine, F2, carefully under controlled conditions, (-45°C, suspension in CFCl3), it is possible to isolate the trifluoride iodine (III) fluoride.
I2 (s) + 3 F2 (g) → 2 IF3 (s) [yellow]
When iodine, I2, is reacted with bromine, Br2, it generates the highly unstable interhalogen species iodine (I) bromide, a solid with a low melting point.
I2 (s) + Br2 (l) → 2 IBr (s)
When Iodine, I2, reacts with chlorine, Cl2, at -80°C with excess liquid chlorine it forms “iodine trichloride”, iodine (III) chloride, I2Cl6.
I2 (s) + 3 Cl2 (l) + I2Cl6 (s) [yellow]
When iodine, I2, reacts with chlorine, Cl2, in the presence of water it forms iodic acid.
I2 (s) + 6 H2O (l) + 5 Cl2 (g) → 2 HIO3 (s) + 10 HCl (g)
- The Reaction of Iodine With Acids
When iodine is reacted with hot concentrated nitric acid (HNO3), it forms iodic acid. The iodic acid crystallizes when it is cooled.
3 I2 (s) + 10 HNO3 (aq) → 6 HIO3 (s) + 10 NO (g) + 2 H2O (l)
- The Reaction of Iodine With Bases
When iodine, I2, is reacted with hot aqueous alkali it produces iodate, IO3–. Only one-sixth of the iodine is converted in this reaction.
3 I2 (g) + 6 OH– (aq) → IO3– (aq) + 5 I– (aq) + 3 H2O
Applications of Iodine
Used In Photography
Silver iodide is a component that can be found in photographic films. Iodine’s first application in the marketplace was in the field of photography. Louis Daguerre was the first person to develop a method for generating images on a piece of metal.
Used As Water Purifier
Iodide has a characteristic that makes it effective as a disinfectant. It is not greatly impacted because of the chlorine by organic content or water pH; nonetheless, the low water temperature significantly limits the iodide’s action as a disinfectant.
Used As Antiseptics
This component can be found in virtually all of the medicinal products that are designed to help clean wounds. Before performing surgery, the skin of the patient might be disinfected using iodine preparations such as povidone-iodine. For the purpose of disinfecting exterior wounds, a solution that contains potassium iodide (KI) and iodine in alcohol is typically applied.
Used In Treatments
Potassium iodide can also be provided as a nutrient to prevent goiter, a thyroid issue that is caused by a lack of iodine. Additionally, adding potassium iodide protects against a form of cognitive disability that is connected with a lack of iodine.
Used In Medical Science
In both its radioactive and not radioactive types, iodine is a crucial element in the field of medicine. Iodide and thyroxin, which is a hormone that contains iodine, are both utilized within the body.
Other Applications
Iodine has a bunch of other applications including use as a catalyst in different industries, it is also used in the production of dyes and printing inks, and also iodine is also used in animal feeds to provide the required iodine.
Health Effects of Iodine
The major and fundamental consequences associated with long-term oral ingestion of excessive quantities of elemental iodide are stated as ironically, hypothyroidism and hyperthyroidism.
Ingestion of an excessive amount of this iodide compound may inhibit the production and discharge of thyroid hormones, which might result in the development of goiter and hypothyroidism.
Iodine, or iodine element, has a vapor that can cause irritation to the lungs and the eyes. Iodine is a hazardous element. Just one milligram per cubic meter is the maximum volume of iodine that could be tolerated in the atmosphere when working with this substance. Iodides of any kind can be hazardous to your health if you consume too much of them.
Environmental Effects of Iodine
Iodine in the air can react with particles of water to form precipitation that can fall into water or soil. Iodine that is present in soils will react with the naturally occurring substances present there and will not move for a very long period. Iodine can be taken up by plants if they are grown in certain soils. As cattle and other animals consume these kinds of vegetation, they will absorb iodine into their systems.
Because of this, the iodine that is found on the water’s surface will transform into vapor and return to the atmosphere. When humans burn fossil fuels for energy, such as coal or fuel oil, they also release iodine gas into the atmosphere. However, the quantity of iodine that is released into the atmosphere as a result of human activity is relatively insignificant in comparison to the amount that is released as a result of evaporation from oceans.
It’s possible that iodine is radioactive. Isotopes that emit radiation are produced by processes that occur naturally in the atmosphere and include chemical reactions. The majority of radioactive iodine isotopes have relatively short half-lives and will rapidly reform into stable iodine compounds if they are not contained.
This isotope is released into the atmosphere by nuclear power reactors, where it is produced as a byproduct of the processing of uranium and plutonium. There has been a significant amount of radioactive iodine released into the atmosphere as a result of accidents that occurred in nuclear power facilities.
References
- https://www.britannica.com/science/iodine
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- https://www.rsc.org/periodic-table/element/53/iodine
- Greenwood NN, Earnshaw A (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- https://byjus.com/chemistry/iodine/
- https://www.lenntech.com/periodic/elements/i.htm
- https://www.chemicool.com/elements/iodine.html
- https://www.chemistrylearner.com/iodine.html
- https://www.livescience.com/37441-iodine.html
