It is the energy required to strip one or more electrons from a neutral atom to create a positively charged ion, which alters the atom’s chemical behavior.
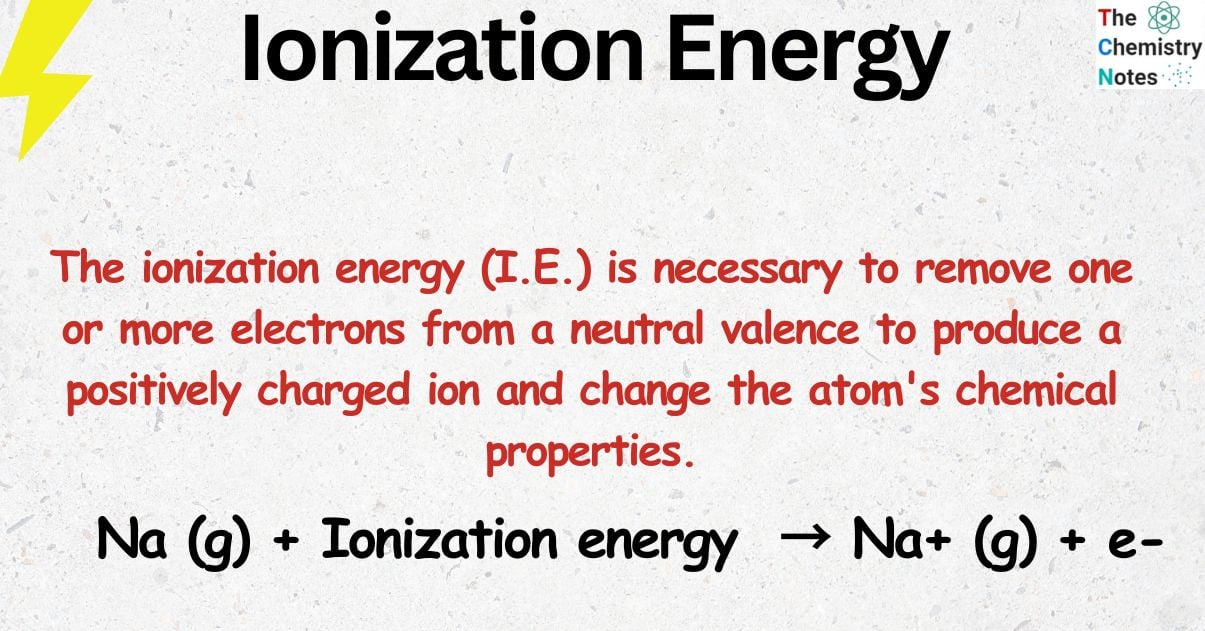
The ionization energy (I.E.) is necessary to remove one or more electrons from a neutral valence to produce a positively charged ion and change the atom’s chemical properties.
It is often expressed in kJ/mol, and the measurement is based on an individual gaseous atom. All elements in the periodic table have a characteristic known as ionization energy, sometimes known as ionization potential.
I.E. can be represented as shown
- X + first ionization energy → X+ + e-
Where
- X is a neutral atom.
- X+ is an ion of atom X with a single positive charge.
- e is an electron with a single negative charge.
Interesting Science Videos
Types of Ionization Energy
First Ionization Energy
The “first ionization energy” of an element refers to the energy needed to remove the outermost valence electron from a neutral atom in the gas phase.
In the equation, it refers to the I.E. required to remove a neutral atom’s first electron, giving an ion with a single positive charge. The process by which the first I.E. of sodium is measured would be represented by the following equation:
Na (g) + energy → Na+ (g) + e–
Second Ionization Energy
The second ionization energy is required to remove the next electron, which is always higher than the first ionization energy because it requires even more energy to remove an electron from a cation than it does from a neutral atom.
For an alkali metal atom, since its loss gives the atom a stable electron shell, removing the first electron is quite simple. Removing the second electron involves forming a new electron shell that is tighter and more closely connected to the atomic nucleus.
The process by which the second I.E. of sodium is measured would be represented by the following equation:
Na (g) + energy → Na2+ (g) + e–
Third Ionization Energy
The “third ionization energy” of an element refers to the energy needed to remove third electron. It is required to form 3+cations.
The process by which the third I.E. of sodium is measured would be represented by the following equation:
Na2+ (g) + energy → Na3+ (g) + e–
All elements have first-ionization energy, even atoms, which do not form positive ions in test tubes.
Throughout the periodic table, the first ionization energy fluctuates constantly. The I.E. decreases from top to bottom in groups and rises from left to right across a period. Therefore, helium has the highest first ionization energy, whereas francium has one of the lowest.
Factors That Affect The Size Of Ionization Energy
Ionization energy is a unit of measurement for the force required to eject a certain electron from the nucleus’s gravitational pull. A high value of I.E. shows a high attraction between the electron and the nucleus. The size of that attraction will be determined as follows:
Distance Of the Electron from the Nucleus:
The distance between the electrons and the nucleus affects the attraction. It rapidly declines with an increase in space. An electron closer to the nucleus is more strongly attracted than one further away.
Nuclear charge:
When the number of protons in the nucleus increases, its positive charge increases, and vice versa. Electrons tend to be strongly attracted to positively charged nuclei.
Number Of Electrons between the outermost electrons and the nucleus:
Let’s take a look at sodium atoms with the electronic structure 2,8,1.
Between the outer electron and the nucleus are the two layers of electrons. The 11 protons in sodium’s nucleus have their effects cut down by the inner electrons. The outer electron only feels a net pull of approximately 1+ from the center. This lessening of the force of the nucleus by inner electrons is known as screening or shielding.
Penetration Effect of Electron
A single atom can contain a variety of subshells, including the s, p, d, and f subshells. As opposed to p-subshell and other subshells, the s-subshell is, as is well known, located closer to the nucleus. In contrast to the other subshells, the s-subshell has a stronger affinity to the nucleus, making it more difficult to remove electrons from it. Therefore, to remove electrons from the s-subshell, additional ionization energy is needed.
Fully and Half-filled electrons
I.E is greater in fully filled orbitals than in half-filled or partially filled orbitals. Partially filled orbitals are less stable than fully filled orbitals.
Calculating Ionization Energy
The following equation can be used to calculate the ionization potential of hydrogen:
E = h c RH ( 1/n2), where
- E is the energy of the electron (or the amount of energy it takes to remove the electron, ionization energy)
- h is Planck’s constant = 6.626 * 10-34 Js (joules seconds)
- c is the speed of light = 3.00 * 108 m/s (meters/second)
- RH is Rydberg constant = 1.097 * 107 m-1 (1/meters)
- n is the principal quantum number (or energy level) of the electron
After applying the values of constants, the equation becomes:
E = (2.18 * 10-18 J)(1/n2)
From here, you can enter the electron’s energy level value to calculate how much power is required to remove it.
Ionization Energy Unit
Ionization energy is typically represented in kcal/mol, kJ/mol, or electron volts (eV) per atom. Mathematically,
A single electron volt (eV) is 3.827*10-20 per atom.
= 3.827 x 10-20 x 4.184 cal per atom (∵ cal=4.184 J)
= 1.602 x 10-19 J per atom
= 1.602 x 10-19 x 6.022 *1023 J/mol
= 96472 J mol-1
= 96.472 kJ mol-1
Ionization Energy Trend On Periodic Table
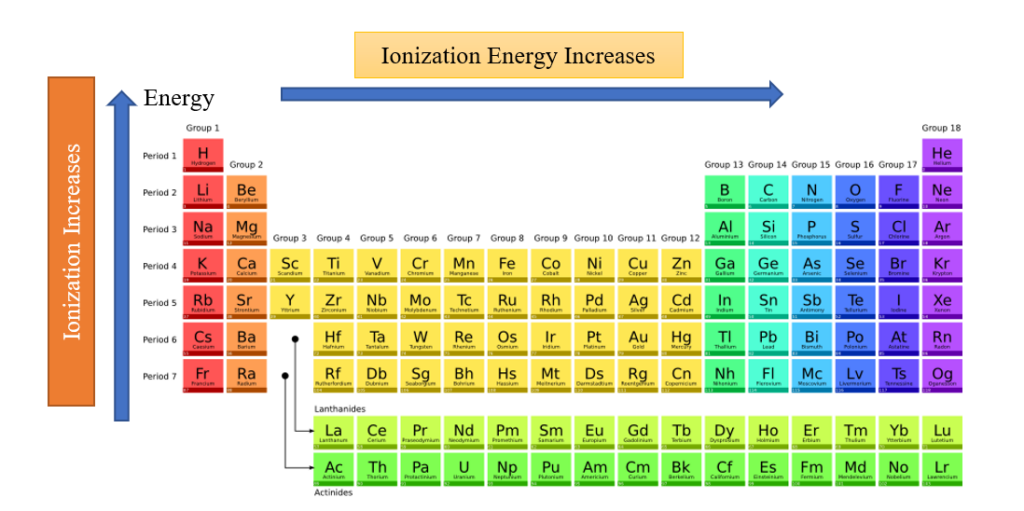
- Moving from left to right across an element period (row), ionization energy normally increases. The reason for this is that the atomic radius decreases as one moves through a period. This occurs as more protons are supplied, increasing the nucleus-electron attraction and pushing the electron shells closer.
- Ionization energy generally decreases moving from top to bottom down an element group (column) as the principal quantum number of the outermost (valence) electron increases moving down. Atoms have more protons moving down a group, which does pull in the electron shells. The outermost electrons are even farther from the nucleus since each row adds a new shell.
- Helium, one of the noble gases, has the highest ionization energy and is found in the upper right corner of the periodic chart. Francium, an alkali metal on the far left side of the chart, has one of the lowest ionization energies.
- Typically, group 2 elements have ionization energy greater than group 13 elements and group 15 elements have greater ionization energy than group 16 elements.
- Groups 2 and 15 have completely and half-filled electronic configuration respectively, thus, it requires more energy to remove an electron from completely filled orbitals than incompletely filled orbitals.
- Alkali metals (IA group) have small ionization energies, especially when compared to halogens or VII A group.
Exception of Ionization Energy Trend on Periodic Table
- Boron has a lower first I.E. than beryllium. Comparing their electronic configuration we can analyze the differences in their ionization energy. Boron with outer electrons in ‘s’ orbital is very close to the nucleus whereas, beryllium releases its first electron from the p-orbital which is comparatively far from the nucleus.
- Nitrogen has three electrons in the p orbital while oxygen has four electrons. Nitrogen should have lower I.E but on contrary, it has lower first ionization energy than oxygen because of the symmetrical distribution of three electrons in the p orbital of a nitrogen atom, by Hund’s rule. The other reason for this exception is electron-electron repulsion.
- Lanthanides and actinides have lower ionization energies due to the presence of an inner ‘f’ electron shell.
1st, 2nd, and 3rd Ionization Energies
- I1 = first ionization energy (energy required to take away an electron from a neutral atom)
- I2 stands for the second ionization energy (energy required to take away an electron from an atom with a +1 charge.
Each succeeding ionization energy is larger than the preceding energy.
This implies I1<I2<I3<…<In will always be true.
Here is an example showing how ionization energy increases as succeeding electrons are ejected.
Mg (g) → Mg+(g) + e– I1 = 738 kJ/mol
Mg+(g) → Mg2+ (g)+ e− I2 = 1451kJ/mol
See first, second, and third ionization energies of elements/ions
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Hydrogen (H) | 1312 | |||||||
| Helium (He) | 2372 | 5250 | ||||||
| Lithium (Li) | 520 | 7297 | 11810 | |||||
| Beryllium (Be) | 899 | 1757 | 14845 | 21000 | ||||
| Boron (B) | 800 | 2426 | 3659 | 25020 | 32820 | |||
| Carbon (C) | 1086 | 2352 | 4619 | 6221 | 37820 | 47260 | ||
| Oxygen (O) | 1402 | 2855 | 4576 | 7473 | 9442 | 53250 | 64340 | |
| Fluorine (F) | 1680 | 3375 | 6045 | 8408 | 11020 | 15160 | 17860 | 92010 |
| Neon (Ne) | 2080 | 3963 | 6130 | 9361 | 12180 | 15240 | ||
| Sodium (Na) | 496 | 4563 | 6913 | 9541 | 13350 | 16600 | 20113 | 25666 |
| Magnesium (Mg) | 737 | 1450 | 7731 | 10545 | 13627 | 17995 | 21700 | 25662 |
References
- F. Albert Cotton and Geoffrey Wilkinson, Advanced Inorganic Chemistry (5th ed., John Wiley 1988) p.1381.
- Lang, Peter F.; Smith, Barry C. “Ionization Energies of Atoms and Atomic Ions”. Journal of Chemical Education. 80 (8).
- Cotton, F. Albert; Wilkinson, Geoffrey (1988). Advanced Inorganic Chemistry (5th ed.). John Wiley. ISBN 0-471-84997-9.
- Housecroft, C.E.; Sharpe, A.G. (November 1, 1993). Inorganic Chemistry (eBook). Inorganic Chemistry. Vol. 3 (15th ed.). Switzerland: Pearson Prentice-Hall. pp. 536, 649, 743.
- https://brilliant.org/wiki/ionization-energy/
- https://chemistrytalk.org/ionization-energy-trend/

