Lanthanide Contraction is an intriguing exception to examine in Chemistry. It is occasionally referred to as the Lanthanoid Contraction. It is applicable to the elements in the Periodic Table’s Lanthanide Series. The atomic numbers of the lanthanide series vary from 57 to 71.
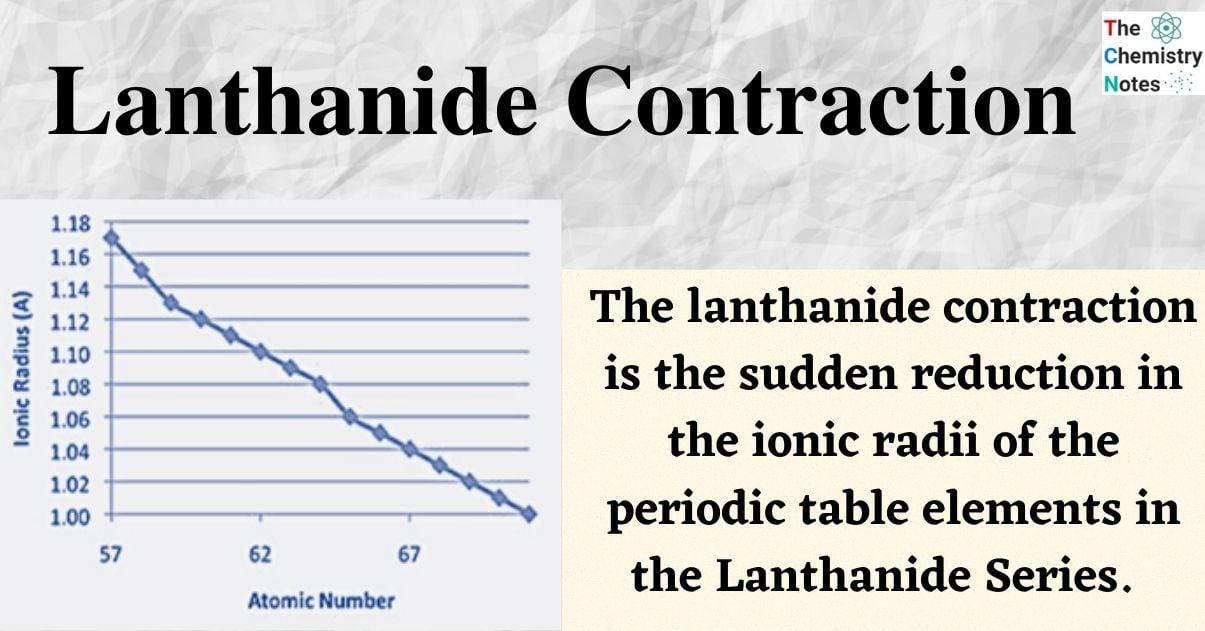
The lanthanide contraction is the sudden reduction in the ionic radii of the elements of the periodic table in the Lanthanide Series.
Interesting Science Videos
Definition: Lanthanide Contraction
Lanthanide contraction is the greater-than-expected decrease in ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to atomic number 71, lutetium, resulting in smaller-than-expected ionic radii for the subsequent elements beginning with 72, hafnium.
The steady reduction in atomic and ionic radii (M3+ ions) of lanthanide elements as the atomic number rises is known as lanthanide contraction. Due to the existence of extra-filled electrons, covalent and ionic radii typically rise as a group descends the periodic table. However, as Z increases, or when traveling from left to right across the lanthanide series, the extra orbital electrons insufficiently shelter the extranuclear charge, forcing all the electrons to be brought closer. This results in a rapid drop in the atomic and ionic radii (M3+ ions).
The 4f electrons only very inadequately protect one another from the rising positive charge of the nucleus, which causes the effective nuclear charge that attracts each electron to continuously increase through the lanthanide elements and cause subsequent decreases in the atomic and ionic radii.
The radius of the lighter lutetium ion, Lu3+, is 0.850 angstroms, compared to 1.061 angstroms for the lanthanum ion, La3+. Because of their similar chemical properties and the fact that the lanthanide contraction maintains these rare-earth ions at a consistent size and that they all often exhibit the +3 oxidation state, at least trace amounts of each one are typically present in every rare-earth material.
Causes of Lanthanide Contraction
A couple of factors account for lanthanide contraction. The 4f subshell is filled in the elements’ electron configuration. Valence electrons are only marginally protected from the positive nuclear charge by the shape of the 4f shell. In essence, the 6s electrons are more frequently in contact with the atomic nucleus than are the 4f electrons. Approximately 10% of the contraction of lanthanide is due to relativistic processes. Because the lanthanide atoms are so massive, the electrons orbit the nucleus at relativistic speeds. They behave as though they are much larger as a result, which also brings them closer to the nucleus.
The following are the reasons for lanthanide contraction:
- Electron compression results from the interaction of a positive nuclear charge with the orbit’s outer shell.
- a rise in the atom count in the elements
- inability of the 11 electrons to protect the 4f electrons 5p6, 5d1, 6s2, and 5s2
- weak shielding between various 4f block components.
- Together with their interaction with positive nuclear charge, all of the 4f block’s constituents have an additive effect that causes them to contract more than the other elements.
- The abrupt decrease in the number of periodic table elements from 57 to 58
Consequences of Lanthanide Contraction
The effects of lanthanide contraction are illustrated in detail by the following points:
- Atomic Size
- Difficulty in the separation of lanthanides
- Effect on the basic strength of hydroxides
- Separation difficulties between second and third transition series elements
- Complex formation
- The ionization energy of d-block elements
Atomic Size
The size of the atom in the third transition series and the atom in the second transition series are remarkably comparable. Examples include the formulas radius of Zr = Hf, radius of Nb = Ta, etc. separating the second and third portions of the transition sequence proved to be problematic.
The elements in the second and third transition series share the same atomic and ionic radii due to lanthanide shrinkage.
Difficulty in the separation of lanthanides
The size and charge of metal ions determine their characteristics. Due to lanthanide contraction, all lanthanide ions have almost the same size and bear the same charge, making their characteristics nearly equal. So, it is exceedingly challenging to separate lanthanides from one another.
Effect on the basic strength of hydroxides
The covalent character of the hydroxides rises and, thus, their basic strength diminishes as the size of lanthanides decreases from La to Lu. Lu (OH)3 is the least basic, while La (OH)3 is the most basic. The ionic size of the lanthanide series gradually lowers from La 3+ to Lu 3+ as a result of lanthanide contraction.
As a result, according to Fajan’s rule, the covalent characteristics of lanthanide metal hydroxide complexes steadily rise from lanthanum to lutetium.
Lu (OH)3 is therefore more covalent than La (OH)3. Now, their fundamental quality deteriorates as the covalent character increases. La (OH)3 is therefore the lanthanide element’s most basic hydroxide, whereas Lu (OH)3 is the least basic.
Separation difficulties between the second and third transition series elements
The size of the third transition series atom is quite similar to the size of the atom in the second transition series.
Examples include a radius of Zr = radius of Hf, the radius of Nb = radius of Ta, etc. difficulties in separating the second and third elements of the transition sequence.
As a result, they have comparable physical and chemical properties. Therefore, it is quite difficult to separate the second and third transition series constituents from one another.
Because of lanthanide contraction, it is extremely difficult, for instance, to separate Zr from Hf.
Complex formation
Because of their smaller size but higher nuclear charge, coordinates are more likely to develop. Complexity rises as La3+ becomes Lu3. Larger and thus having a lower charge-to-radius ratio are lanthanides that display the 3+ oxidation state. Due to this, lanthanides’ capacity to form complexes relative to d-block elements is diminished. Even so, they create compounds with potent chelating substances such as EDTA, -diketones, oxime, etc. P-complexes are not formed by them.
Ionization energy and electronegativity
The ionization energy of 5d elements is substantially higher than that of 4d and 3d because the nuclear charge attracts electrons at a much higher rate. Except for Pt and Au, all elements in the 5d series have filled the s-shell.
Hafnium and rhenium have the same ionization energy, and after that, it rises with the number of shared d-electrons, with gold and iridium having the highest ionization energy. They become less chemically reactive as a result. For instance, the sixth-period elements, such as gold (Au), platinum (Pt), mercury (Hg), etc., have very low chemical reactivity. From La to Lu, electronegativity increases.
Density variation
Between the second and third transition metal series, there is a density difference. Due to lanthanide contraction, the density of the second and third transition metal series is significantly higher. Since the atomic volume of two separate elements from the 4d and 5d series belonging to the same group is nearly identical, the atomic mass of elements from the 5d series is significantly higher.
Shielding and its Effects on Atomic Radius
The 4f electrons’ ineffective shielding produces the Contraction. The shielding effect is the phenomenon where the outer-shell electrons are protected from nuclear charge by the inner-shell electrons. Therefore, when the shielding is less effective, the positively charged nucleus will have a stronger attraction to the electrons, which will cause the atomic radius to decrease as the atomic number rises. The shielding is greatest in the s orbital, lowest in the f orbital, and intermediate between the two in the p and d orbitals, with p being higher than d.
By contrasting elements with and without f electrons in the d block orbital, the Lanthanide Contraction can be visualized. Such elements include Pd and Pt. While Pt contains 5d and 4f electrons, Pd only has 4d electrons. The atomic radius of these two elements is similar. Shielding and lanthanide contraction are to blame for this. As more electrons and protons are added, we would anticipate Pt to have a substantially bigger radius, but this is not the case since the 4f electrons are not very good at shielding. When the shielding is inadequate, there will be a higher nuclear charge, which will draw the electrons in closer and cause a radius that is lower than anticipated.
Effect of Lanthanide Contraction in Periodic Table
Well, as mentioned above, Lanthanide contraction applies to the elements of the lanthanide series only. And, there are a total of 14 elements in the series.
| Atomic Number | Element | Ln3+ radius (pm) (6-coordinate) |
| 57 | Lanthanum | 103 |
| 58 | Cerium | 102 |
| 59 | Praseodymium | 99 |
| 60 | Neodymium | 98.3 |
| 61 | Promethium | 97 |
| 62 | Samarium | 95.8 |
| 63 | Europium | 94.7 |
| 64 | Gadolinium | 93.8 |
| 65 | Terbium | 92.3 |
| 66 | Dysprosium | 91.2 |
| 67 | Holmium | 90.1 |
| 68 | Erbium | 89 |
| 69 | Thulium | 88 |
| 70 | Ytterbium | 86.8 |
| 71 | Lutetium | 86.1 |
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- https://byjus.com/jee/lanthanides/
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/4_fBlock_Elements/The_Lanthanides/aLanthanides%3A_Properties_and_Reactions/ Lanthanide_ Contraction
- https:// kgghosh1990.medium.com/lanthanide-contraction-definition-causes-consequences-in-chemistry-f301029dac7a
- https://unacademy.com/content/neet-ug/study-material/chemistry/ a-brief-note -on-lanthanide-contraction/
- Cotton, F. Albert; Wilkinson, Geoffrey (1988). Advanced Inorganic Chemistry (5th ed.). New York: Wiley-Interscience. ISBN 0-471-84997-9.
- Goldschmidt, Victor M. (1925). “Geochemische Verteilungsgesetze der Elemente”, Part V “Isomorphie und Polymorphie der Sesquioxyde. Die Lanthaniden-Kontraktion und ihre Konsequenzen”. Oslo.
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.
