A Lewis structure is a diagram that depicts the chemical bonds that exist between atoms in a molecule and their valence electrons, also known as lone pairs of electrons. The diagram is also known as a Lewis dot diagram, a Lewis dot formula, or an electron dot diagram. A Lewis structure depicts how electrons are grouped around atoms but does not explain how electrons are shared between atoms, how chemical bonds occur, or the shape of a molecule.
Lewis structures are named after Gilbert N. Lewis, who first proposed the concept in his 1916 paper “The Atom and the Molecule.”
Interesting Science Videos
What is Lewis Structure?
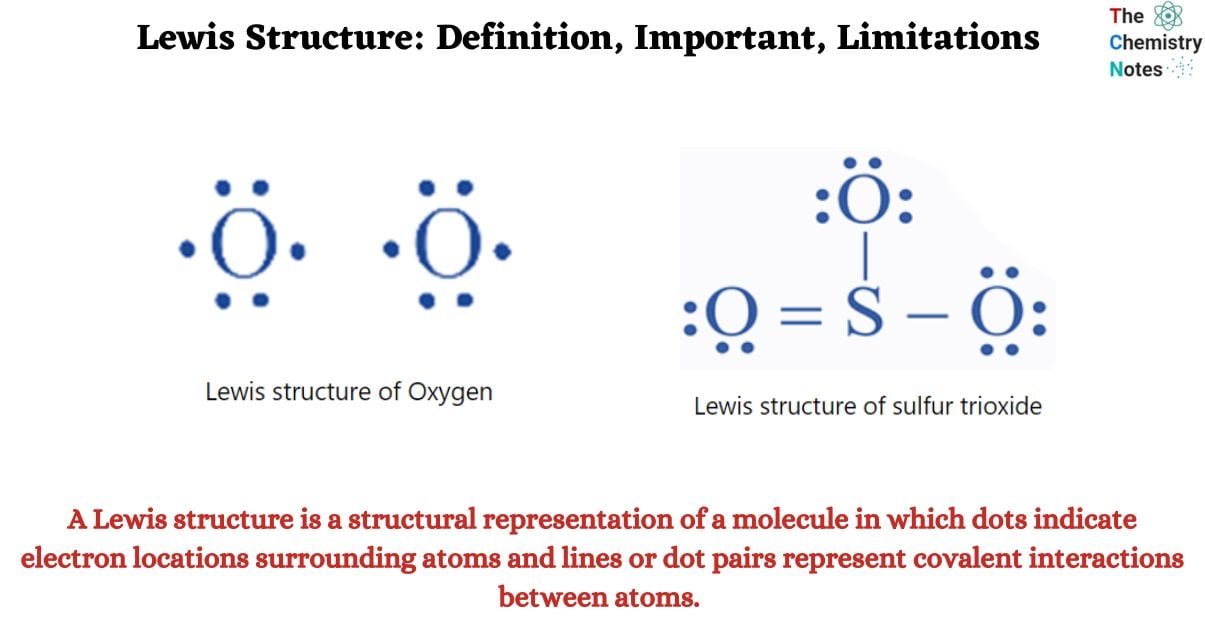
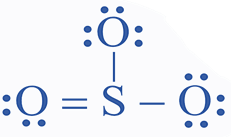
A Lewis structure is a structural representation of a molecule in which dots indicate electron locations surrounding atoms and lines or dot pairs represent covalent interactions between atoms.
The objective of drawing a Lewis dot structure is to identify lone electron pairs in molecules in order to help determine chemical bond formation. Lewis structures may be created for molecules with covalent bonds as well as coordination compounds. The reason is because electrons are shared in a covalent link. In an ionic bond, one atom contributes an electron to the other.
Lewis Structure Representation
A Lewis structure is based on the octet rule, which states that atoms share electrons in order for each atom to have eight electrons in its outer shell. An oxygen atom, for example, contains six electrons in its outer shell. These six dots are placed in a Lewis structure such that one atom contains two lone pairs and two single electrons. The two pairs would be opposite each other around the O sign, whereas the two single electrons would be on opposing sides of the atom.
Single electrons are generally written on the side of an element symbol. An example of inappropriate placement would be four electrons on one side of the atom and two on the other. When oxygen forms a link with two hydrogen atoms to make water, each hydrogen atom has one dot for its lone electron. The water electron dot structure displays single oxygen electrons sharing space with single hydrogen electrons. Because the molecule possesses a stable octet, all eight locations for dots surrounding oxygen are filled.
Lewis Structure Components
For molecules and complexes, Lewis structures are illustrated. A Lewis structure is made up of the following components:
- Symbols for elements
- Dots that represent valence electrons
- Chemical bond lines (one line for a single bond, two lines for a double bond, etc.)
- The dots and lines follow the octet rule.
- If the structure has a net charge, it is surrounded by brackets, and the charge is given in the top righthand corner.
It should be noted that the phrases “Lewis structure” and “electron dot structure” are sometimes used interchangeably. Technically, they are not the same. A Lewis structure employs lines to represent chemical bonds, whereas an electron dot structure employs simply dots.
How to Draw a Lewis Structure
- Determine the total number of valence electrons in the molecule for all atoms.
This is the total of the valence electrons in each atom in a neutral molecule. Except for helium and metals, the number of valence electrons for an element is typically the same as its group number on the periodic table. Subtract one electron for each positive charge or add one electron for each negative charge if the molecule has a charge. For example, in NO3–, the nitrogen atom has 5 electrons and the oxygen atoms have 3 x 6 = 18 electrons, plus one valence electron for the net charge, for a total of 24 valence electrons (5 + 18 + 1).
- Draw the skeletal structure of the molecule.
Assume the atoms are joined by single bonds at this moment. The atom with the greatest bonding sites is usually the central atom (thus carbon would be central above oxygen).
Determine the number of electrons required to meet the octet rule. Hydrogen and helium have two electrons in their valence electron shells. The valence shell of other atoms fills with 8 electrons up to period 4 of the periodic table. Each chemical bond requires two electrons, thus build each link between atoms in the skeletal structure with two valence electrons. Six electrons were employed to draw the single bonds for the skeleton of NO3–. So just 18 electrons are left. Distribute the atoms starting with the most electronegative.
- Distribute the valence electrons that remain.
In order to implement the octet rule, illustrate these non-bonding electrons as dots surrounding the atoms.
- Draw the molecule’s chemical bond.
Make double or triple bonds if all of the octets aren’t filled. To do this, take a lone pair of electrons from an electronegative atom and form a bonding pair with an electropositive atom that lacks electrons.
- Check that each atom has the lowest formal charge possible.
Don’t break the octet rule. The formal charge is equal to the number of valence electrons divided by half the number of bonding electrons divided by the number of lone electrons. So, for each single-bonded oxygen, the equation is 6 – 1 – 6 = -1; for nitrogen, the equation is 5 – 4 – 0 = +1; and for double-bonded oxygen, the equation is 6 – 2 – 4 = 0. The net formal charge is -1 + -1 + 1 + 0 = -1 because there are two single-bonded oxygen atoms, one nitrogen atom, and one double-bonded oxygen atom. Indicate the formal charges individually, or put a bracket around the structure and superscript – or -1.
Some Examples of Lewis Structures

Lewis Structures Important
- Lewis structures, which include several atoms that fully or partially fill their valence shell, aid in the description of valence, chemical bonding, and oxidation states.
- With eight valence electrons, lighter elements behave in a manner that is quite similar to that which the structures describe.
- The behavior of carbon, hydrogen, and oxygen is crucial in organic chemistry and biology, therefore they are very useful in these fields. Lewis structures are used to forecast geometry, reactivity, and polarity even though they don’t always display geometry.
Limitations of Lewis Structure
- When molecules, like the lanthanides and actinides, include atoms with more than eight valence electrons, they don’t function properly.
- Compounds that are inorganic and organometallic use bonding schemes that go beyond what Lewis structures can explain.
- Molecular orbitals in particular might be completely delocalized. Aromaticity is not taken into account by Lewis structures.
- Lewis structures might lead to inaccurate predictions concerning bond length, magnetic properties, and bond ordering, even with lighter molecules (O2, ClO2, NO), since the anticipated structures deviate sufficiently from the observed behavior.
References
- https://testbook.com/chemistry/lewis-dot-structure
- https://infinitylearn.com/surge/blog/neet/important-topic-of-chemistry-lewis-structure/
- IUPAC (1997). “Lewis formula”. Compendium of Chemical Terminology (the “Gold Book”) (2nd ed.). Blackwell Scientific Publications. ISBN 0-9678550-9-8.
- Lewis, G. N. (1916), “The Atom and the Molecule”. J. Am. Chem. Soc. 38 (4): 762–85. doi:10.1021/ja02261a002
- https://www.studysmarter.co.uk/explanations/chemistry/ionic-and-molecular-compounds/limitations-of-lewis-dot-structure/
- https://sciencenotes.org/how-to-draw-a-lewis-structure/
