Ammonia, or NH3, is a colorless, pungent gas made up of one nitrogen and three hydrogen atoms. It is a significant chemical molecule with numerous applications in agriculture, industry, and biology. Lewis structure of NH3 and it’s geometry are essential for understanding its characteristics and behavior in different situations. A molecule’s Lewis structure facilitates understanding of electron geometry, molecular geometry, polarity, and other characteristics. It represents the arrangement of valence electrons around the atoms in the molecule.
Interesting Science Videos
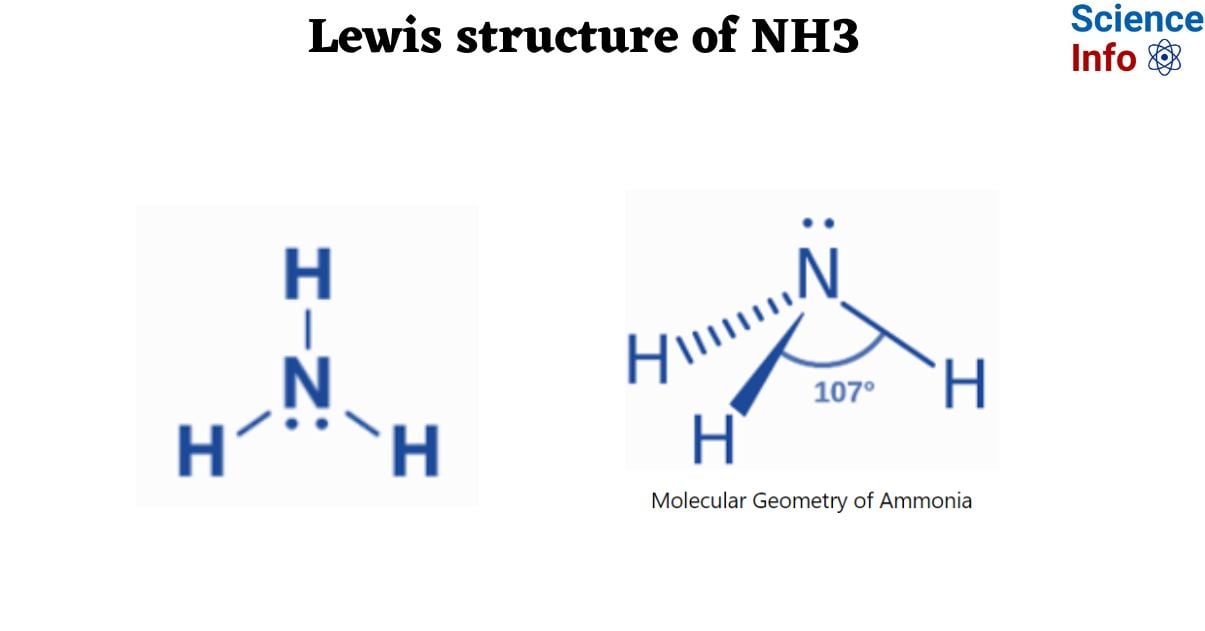
Lewis Structure of NH3
The Lewis structure of NH3 (Ammonia) contains a Nitrogen atom (N) in the center, surrounded by three Hydrogen atoms (H). Each Nitrogen atom (N) and Hydrogen atom (H) has three single bonds. The Nitrogen atom (N) has one lone pair.
How to Draw Lewis Structure of NH3
Step 1: Determine the total number of valence electrons in the NH3 molecule.
To determine the total valence electrons in an NH3 molecule, first determine the valence electrons present in the nitrogen and hydrogen atoms.
The nitrogen atom has 5 valence electrons, whereas the hydrogen atom has only 1 valence electron. As a result, the total number of valence electrons in NH3 is computed using the formula:
Total valence electrons = 1 nitrogen atom’s valence electrons + 3 hydrogen atoms’ valence electrons = 5 + 1*(3) = 8.
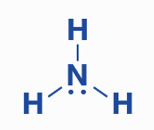
Step 2: Find and position the atom in the center that has the lowest electronegativity.
In theory, the less electronegative atom remains at the center. Because they are more likely to share electrons with adjacent atoms, hydrogen atoms are less electronegative than nitrogen (N) electronegativity values. Hydrogen, on the other hand, is an exception. In the Lewis diagram, hydrogen is always on the outside. Because hydrogen atoms can only make one bond, we may approximate the position of the four atoms.
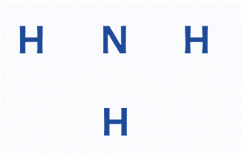
Step 3: Use a single bond to connect the outer atoms to the center atom, and then insert electron pairs between the atoms.
In this stage, use a single bond to connect all of the outside atoms to the center atom. A single bond is formed by an electron pair between two atoms. Six of the eight electrons will be employed in atom pairs. There are one Lone pair electron. As a result, there is just one lone electron pair to be distributed. The hydrogen atoms are the outer atoms in the ammonia Lewis structure, and each cannot hold more than two electrons in its last shell. As a result, the final electron pairs were drawn on the center of the nitrogen atom.
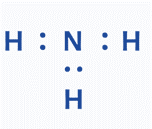
Step 4: Verify that the structure is stable

The lower the formal charge on atoms, the more stable the Lewis diagram. To determine an atom’s formal charge. Use the following formula:
Formal charge = Valence electrons (V) – Lone electron pair (L) – Bond electron pair (B)/2.
Formal charge=1-0-2/2=0 for hydrogen atom
Formal charge=5-2-6/2=0 for nitrogen atom
Because the overall formal charge of NH3 is zero, the above Lewis structure is the most appropriate, dependable, and stable.
Molecular Geometry of NH3
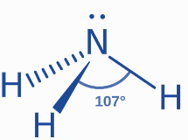
Lone Pair: The molecule of ammonia (NH3) is made up of one nitrogen (N) atom and three hydrogen (H) atoms. The nitrogen atom has five valence electrons and needs three more to make an octet. The hydrogen atom contains one valence electron and needs another to complete its 1s valence shell. Nitrogen and hydrogen will create a single covalent bond by sharing electrons, according to the Lewis structure. Ammonia has three N-H bonds and one electron pair on nitrogen.
Bond Pair: Compared to lone pairs, bond pairs function differently. Bond couples are drawn to both nuclei. Because of the polarity of the N-H bond, it will be closer to nitrogen than hydrogen. The lone pair, on the other hand, will be closer to its atom’s nucleus. The effective solid angle will be larger than the solid angle of the bond pairs.
VSEPR: VSEPR theory is the most effective way for studying molecule shape. Ammonia has a tetrahedral electron shape. Because of the lone pair, its molecular geometry is deformed tetrahedral. According to VSEPR theory, the lone pair will repel the bond pair. The electrical repulsion diminishes in the following order.
lone pair – lone pair > lone pair-bond pair > bond pair-bond pair
As a result, the N-H bonds will bend inward, away from the lone pair. With a bond angle of 107°, ammonia will form a trigonal pyramidal structure. This angle is less than 109°, which is the optimal tetrahedral bond angle. The VSEPR notation is AX3E1.
Video on Lewis Structure of NH3
References
- https://terpconnect.umd.edu/~wbreslyn/chemistry/Lewis-Structures/lewis-structure-for-NH3.html
- https://pediabay.com/nh3-lewis-structure/
- https://www.thegeoexchange.org/chemistry/bonding/Lewis-Structures/NH3-Lewis-structure.html
- https://www.chemistryscl.com/general/NH3-lewis-structure/index.php
- https://www.chemicalbook.com/article/how-do-we-determine-the-lewis-structure-for-the-nh3-molecule.htm

