A complex’s ligands can be exchanged entirely or partially for other ligands. This kind of reaction is a substitution reaction. It happens if the new complex formed is greater stable than the original complex. Ligand substitution is typically the first reaction encountered in organometallic chemistry. In general, ligand substitution is the substitution of one ligand for another with no change in the oxidation state at the metal center. The incoming and outgoing ligands can be L- or X-type, but if the ligand type changes, the charge of the complex changes.
Ligand Exchange- (Ligand Substitution)
This substitution occurs when one ligand in a complex is replaced by another.
This results in the formation of a new complex that is more stable than the original. Others can partially or completely replace the ligands in the original complex. If the ligands are of similar size, there are no changes in coordination number or complex geometry.
However, if the ligands are of different sizes, such as water ligands and chloride ligands, the coordination number and geometry of the complex will change.
Ligand substitution is defined by a set of mechanisms that are divided into associative (A) and dissociative (D) extremes.
The incoming ligand first forms a bond with the metal, then the departing ligand takes its lone pair and leaves. The order of events is reversed at the dissociative extreme—the departing ligand leaves first, followed by the incoming ligand. For 16-electron complexes (such as d8 complexes of Ni, Pd, and Pt), associative substitution is common, whereas dissociative substitution is the norm for 18-electron complexes. However, the reality is frequently more complicated than these extremes. Evidence for simultaneous dissociation and association is available in some cases, and this mechanism has been given the name interchange (IA or ID).
Copper (II) ion complexes can be used to demonstrate ligand substitution reactions.
Ligand Substitution in copper (II) complexes
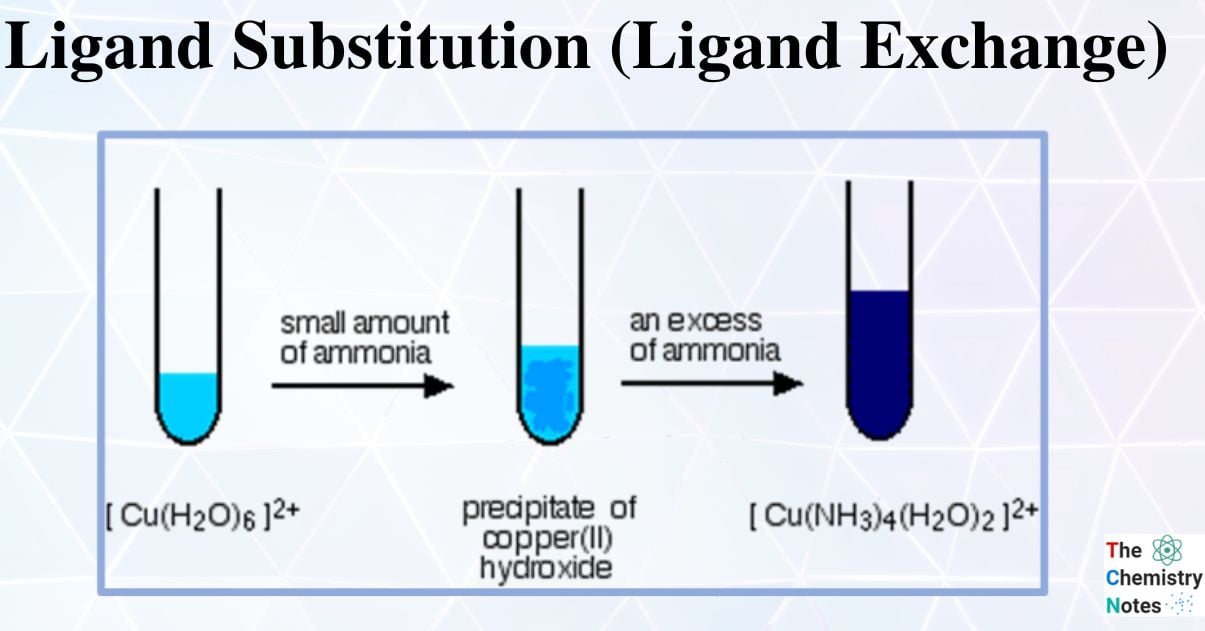
When a transition element ion is present in the solution, it is assumed to exist as a hexaaqua complex ion (i.e. it has six water ligands attached to it). When we say Cu2+ (aq), we’re really talking about the complex ion [Cu (H2O)6] 2+
The complex ion [Cu (H2O)6]2+ (aq) is blue in color.
![Blue color complex of [Cu (H2O)6]2+](https://scienceinfo.com/wp-content/uploads/2022/11/image-13.png)
Substitution by Hydroxide ligands
- A light blue precipitate is formed when sodium hydroxide (NaOH) solution is added dropwise.
- Two water ligands were partially substituted by two hydroxide ligands.

Substitution by Concentrated Ammonia
The pale blue precipitate dissolves when excess concentrated ammonia (NH3) solution is added, forming a deep blue solution. Hence, partial ligand substitution has occurred once more.

The first reaction can also be accomplished by adding concentrated ammonia solution drop by drop to copper sulfate solution or by adding a dilute ammonia solution.
When excess ammonia is added, the pale blue precipitate will dissolve and form a deep blue solution.
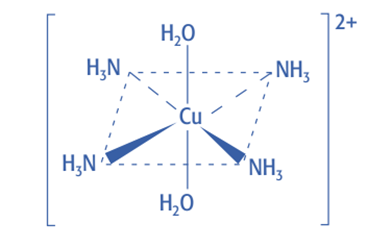
![Deep-blue color complex [Cu (H2O)2(NH3) 4] 2+](https://scienceinfo.com/wp-content/uploads/2022/11/image-12.png)
Substitution by Chloride
The water ligands in [Cu (H2O)6]2+ can also be substituted by chloride ligands when concentrated hydrochloric acid is added (HCl)
The blue solution turns yellow due to the complete substitution of the water ligands.
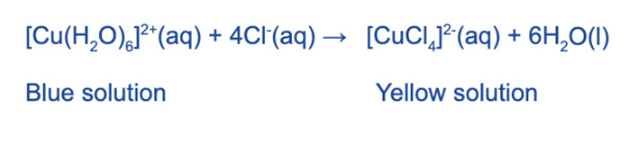

Because chloride ligands are larger than water ligands, only 4 will fit around the central metal ion, so the coordination number has been reduced from 6 to 4. The complex’s geometry has also changed from octahedral to tetrahedral.
Because this is a reversible reaction, some of the [Cu (H2O)6]2+ complex ions will remain in the solution. The reaction mixture will turn green due to the combination of blue and yellow solutions.
When water is added to the solution, the chloride ligands are displaced by the water molecules, and the [Cu (H2O)6]2+ (aq) ion and blue solution return.
Ligand Substitution in Cobalt (II) complexes
The complex ion [Co (H2O)6]2+(aq) is pink in color.
A blue precipitate is formed when sodium hydroxide (NaOH) solution is added dropwise.
Two water ligands were partially substituted by two hydroxides (OH–) ligands.

Substitution by Concentrated Ammonia
When an excess of concentrated ammonia solution is added to [Co (H2O)6]2+, a brown solution is formed. Because the ammonia was added in excess and not dropwise, no precipitate will form in this case. The water ligands have been completely replaced by ammonia ligands.
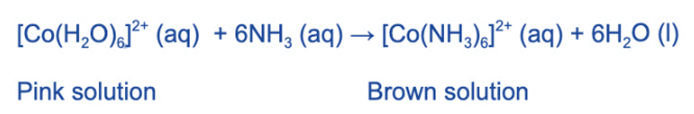
Substitution by Chloride
The water ligands in [Co (H2O)6]2+ can also be substituted by chloride ligands when concentrated hydrochloric acid is added.
The pink solution turns blue due to the complete substitution of the water ligands.
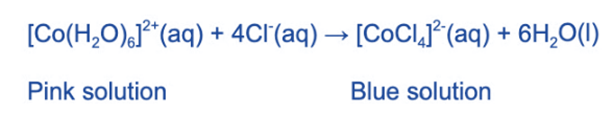
The coordination number has changed from 6 to 4, as with [Cu (H2O)6]2+above, because the chloride ligands are larger than the water ligands, and only 4 will fit around the central metal ion.The complex’s geometry has also changed from octahedral to tetrahedral.
When water is added to the solution, the chloride ligands are displaced by the water molecules, and the [Co (H2O)6]2+ (aq) ion and pink solution return.
Chelate Effect
A more stable complex is formed when bidentate and multidentate ligands replace monodentate ligands. This is known as the chelate effect.
When ligand exchange reactions take place, the strength of the ligand bonds that are broken is very similar to the strength of the new bonds that are formed. This means that for many ligand substitution reactions, the enthalpy changes, H, are very small.
The increased stability is primarily explained by an increase in entropy, S. As more product molecules are formed, there is more disorder (increased entropy) in the products.
EDTA ⁴⁻ replacing water ligands
Consider the reaction in which an EDTA⁴⁻ ligand replaces water ligands.
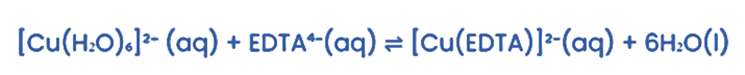
There are two reactant particles and seven product particles. This increase in product particles greatly increases the entropy, ∆S.
The Gibb’s free energy, ∆G, will be negative because the change in entropy, S, is positive and the change in enthalpy, ∆H, is small.
∆G = ∆H – T∆S
This implies that the reaction’s equilibrium will be far to the right.
As a result, multidentate and bidentate ligands form more stable complexes than monodentate ligands.
References
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General chemistry: principles and modern applications (8th ed.). Upper Saddle River, N.J: Prentice Hall
- Housecroft, C. E. and Sharpe, A. G. (2005) Inorganic Chemistry, 2nd ed, Pearson Prentice-Hall
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Organometallic_Chemistry_(Evans)/Fundamentals_of_Organometallic_Chemistry/Ligand_substitution#:~:text=In%20general%2C%20ligand%20substitution%20involves,if%20the%20ligand%20type%20changes.
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/6-inorganic-chemistry-a-level-only/6-3-transition-element-complexes-isomers-reactions–stability-a-level-only/6-3-1-ligand-exchange/
- https://studymind.co.uk/notes/ligand-substitution-reactions/
- Cotton, F. A. and Wilkinson, G. (1988) Inorganic Chemistry, 5th ed., Wiley, pp. 625–627. ISBN 978-0-471-84997-1.
