Metals are naturally occurring substances found in the crust of the earth. They are typically found in the form of metal ores and are linked to numerous other elements as well as one another. Metals are typically bright or lustrous. Since metals are inorganic, they are composed of materials that were never alive. Since metal is so strong and resilient, it’s employed in a lot of products. These are used to make kitchen utensils, cars, satellites, and other things.
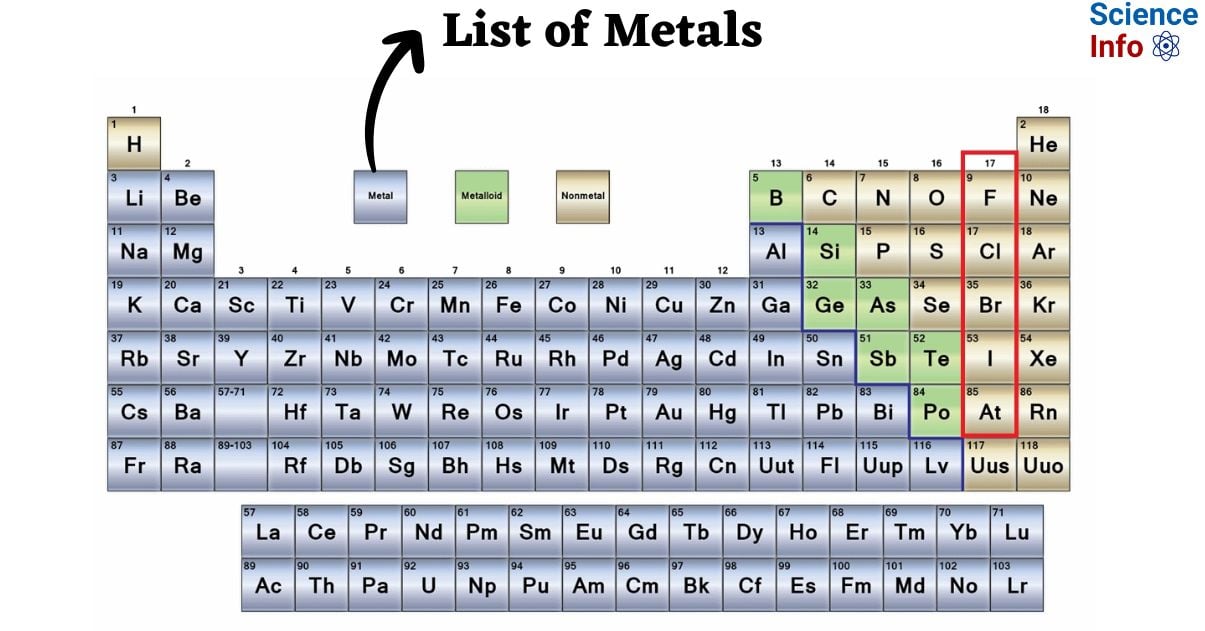
Metals comprise the vast majority of the elements on the periodic table. On the periodic table, they are arranged in a group towards the left of the center. The metals include actinides, lanthanides, transition metals, alkali metals, and alkaline earths. Metalloids, nonmetals, halogens, and noble gases are examples of elements that are not metals.
Interesting Science Videos
List of Metals
Alkali Metals: Alkali metals are classified as IA on the far left side of the periodic table. Due to their +1 oxidation state and often lower density than other metals, these elements are extremely reactive. These elements are present in compounds due to their high degree of reactivity. Only hydrogen exists in nature as a pure element, in the form of diatomic hydrogen gas.
Alkaline Earth Metals: Alkaline earth metals are classified as group IIA of the periodic table, which is the second column of elements. All alkaline earth metal atoms have an oxidation state of +2. These elements, like alkali metals, exist in compounds rather than in their pure form. Alkaline earths are less reactive than alkali metals. Group IIA metals are hard, lustrous, malleable, and ductile.
Basic Metals: The basic metals exhibit the qualities commonly associated with the term “metal.” They conduct heat and electricity, have a metallic shine, and are often dense, malleable, and ductile. However, several of these elements exhibit nonmetallic properties. For example, one allotrope of tin behaves more like a nonmetal. Most metals are hard, but lead and gallium are soft. With a few exceptions, these elements generally have lower melting and boiling points than transition metals.
Transition Metals: The transition metals are distinguished by their partially filled d or f electron subshells. Because the shell is incompletely filled, these elements exhibit numerous oxidation states and frequently form colorful complexes. Some transition metals, such as gold, copper, and silver, exist in their pure or natural form. Lanthanides and actinides can only be found in naturally occurring chemicals.
| Classification | Elements |
| Alkali Metals | Hydrogen (in metallic state), Lithium, Sodium, Potassium, Rubidium, Cesium, Francium |
| Alkaline Earth Metals | Beryllium, Magnesium, Calcium, Strontium, Barium, Radium |
| Basic Metals | Aluminum, Gallium, Indium, Tin, Thallium, Lead, Bismuth, Nihonium, Flerovium, Moscovium, Livermorium (Tennessine may behave like a metalloid or metal) |
| Transition Metals | Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Yttrium, Zirconium, Niobium, Molybdenum, Technetium, Ruthenium, Rhodium, Palladium, Silver, Cadmium, Lanthanum, Hafnium, Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Mercury, Actinium, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium, Roentgenium, Copernicium, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium, Mendelevium, Nobelium, Lawrencium |
Physical Properties of Metals
- Metals are bright and malleable. They are also ductile and good conductors of heat and electricity.
- The metals are solid at normal temperatures. Mercury is the only exception; it can be liquid at normal temperatures (Gallium is liquid on warm days).
- Metals, such as copper, silver, and gold, can reflect light off their surfaces when polished.
- Metals are malleable, which means they may be hammered and molded into thin sheets known as foils. For example, a gold bar may be crushed into a fine sheet large enough to fill a football field.
- Ductility: Metals can be drawn in wires. For example, 100 grams of silver might be converted into a fine wire measuring approximately 200 meters.
- Hardness: All metals are hard, except for potassium and sodium, which are soft and easily cut with cutlery.
- Metals typically have one to three electrons in their outermost shell.
- Conduction Metals are excellent conductors because of their free electrons. Copper and silver are the two best conductors of electricity and heat. Lead is the absolute worst heat conductor. Bismuth, mercury, and iron are all poor conductors.
- Metals have the highest density and are quite heavy. Osmium and Iridium have the highest density, whereas lithium is at the bottom of the density range.
- Metals have extremely high melting and boiling points. Tungsten has the highest melting and boiling points, while mercury has the lowest. Potassium and sodium have low melting points.
Uses of Metals
Construction Industry: Metals such as iron, steel, and others are the most commonly utilized materials in the construction of structures and dwellings. They are also metals utilized in architectural applications. Water pipes and roofing are made from metals such as lead. Tin, zinc, copper, and aluminium are used in roofing. Iron is known for its structural properties and is utilized in the form of cast iron, wrought iron, or steel.
Electronic Industry: Because of their excellent electrical conductivity, alloys containing gold, silver, and platinum are popular candidates for electronic connections. However, other metals, such as silver, are prone to deterioration and are not utilized in the electrical sector.
Applications in Medicine: Metals such as gold, stainless steel, and cobalt-chrome are used in the medical field. They also act as cofactors or prostheses in enzymes, catalyzing certain processes and playing critical roles. The necessary metals for humans are sodium, potassium, magnesium, copper, vanadium, chromium, manganese, iron, cobalt, nickel, zinc, molybdenum, and cadmium. Their unique qualities provide them an advantage, and hence they are used in the pharmaceutical sector.
Machinery, Refractory, and Automobiles: Metals are most typically utilized in equipment, refractory products, and cars. They are employed in the production of automobiles, trains, airplanes, rockets, and so on. The most prevalent metals utilized here are iron, aluminum, and steel. Kitchen utensils are made from steel, aluminum, and copper.
Decorative Products: Platinum, gold, and silver are precious metals with significant economic worth and are commonly used in jewelry sets and decorative pieces.
Other Uses: Metals are also employed in military applications to manufacture guns, munitions, and ammunition. Galvanizing uses metals such as aluminum and other alloys to prevent rusting.
References
- https://testbook.com/chemistry/uses-of-metals
- https://byjus.com/chemistry/metal/
- Helmenstine, Anne Marie, Ph.D. “Metals: List of Elements.” ThoughtCo, Aug. 27, 2020, thoughtco.com/metals-list-606655.
- https://sciencenotes.org/list-metals/
