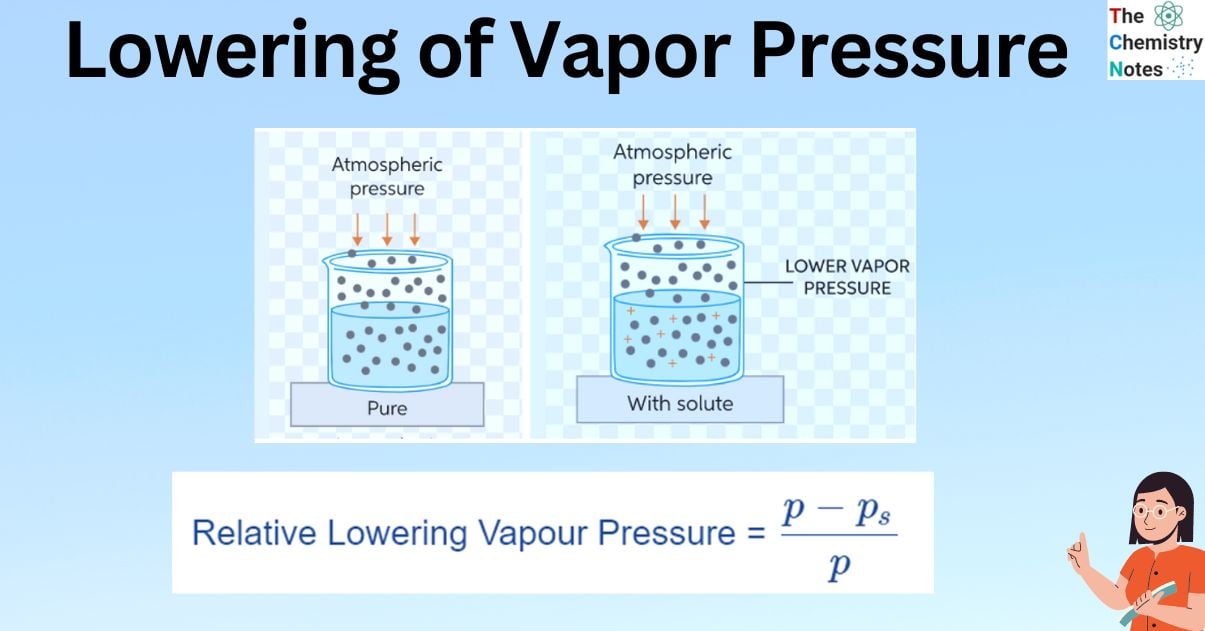
When a non-volatile solute is added to a solvent, some of the solute molecules take up surface space and decrease the rate of evaporation. Since they are non-volatile, these solutes are difficult to remove. The vapor pressure of the solution is thus reduced which is referred to as the lowering of vapor pressure. In such cases, the vapor pressure of the solution is always lower than that of the pure solvent. This decrease in vapor pressure is proportional to the amount of non-volatile solute added to the solution, independent of its type, and is thus one of the colligative properties.
Interesting Science Videos
Relative Lowering of Vapor Pressure (Raoult’s Law)
When a liquid is in an equilibrium state at a specific temperature, the pressure that the vapours exert on it is known as vapour pressure.
Take a pure liquid as an example. The molecules of the pure liquid are present on the liquid’s surface. Only pure liquid (solvent) particles make up the vapour that is suspended above the liquid. If a non-volatile solute is added to this pure liquid, the vapour pressure of the solution is discovered to be lower than that of the pure liquid at the given temperature because the solute molecules are non-volatile.
Because both pure liquid and solute molecules were present on the liquid’s surface after the solute was introduced to the pure liquid (solvent), the vapour pressure decreased. There are fewer solvent molecules that escape into the gas phase, which lowers the pressure. Because both pure liquid and solute molecules were present on the liquid’s surface after the solute was introduced to the pure liquid (solvent), the vapor pressure decreased. The number of solvent molecules that escape into the gas phase is reduced, lowering the pressure exerted by the solvent molecules in the gaseous phase. This is referred to as a relative lowering of vapor pressure.
The relative lowering of pressure and lowering of pressure are colligative property.

Raoult’s Law
Raoult’s law makes it simple to see why vapor pressure is lowering. According to Raoult’s Law, the relative lowering of vapor pressure must be equal to the mole fraction of the solute. It is determined by Ostwald–Walker method.
Mathematical Representation of Raoult’s Law
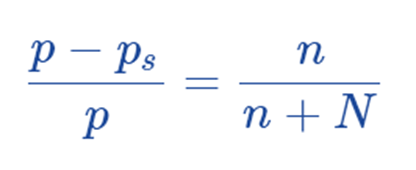
where,
P – Ps = Lowering of Vapor Pressure
n = number of moles of the solute
N = number of moles of the solvent
In other words;
Raoult’s Law states that the partial vapor pressure of the solvent in the solution at particular temperature is equal to the vapor pressure of the pure solvent multiplied by its mole fraction of the solution.
Psolution (Ps) = Xsolvent * P
where,
Psolution= Vapor Pressure of Solvent in solution = Ps
P = Vapor Pressure of Pure Solvent
Xsolvent = Mole fraction of solvent
Derivation of Raoult’s Law
The vapor pressure of a pure solvent is determined by the number of molecules that evaporate off its surface. When a non-volatile solute is dissolved in solution, the presence of solute molecules on the surface blocks a portion of the surface, preventing evaporation.
When a nonvolatile solute lowers the vapor pressure, the particles of the solute prevent solvent molecules from escaping from the solution’s surface. This eventually leads to a decrease in vapor pressure. The vapor pressure of the solution is thus determined by the number of molecules of the solvent present at any given time on the surface, which is proportional to the mole fraction. Therefore,
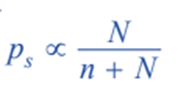
Where, n = number of moles of solute
N = number of moles of solvent
Now the equation can be written as;
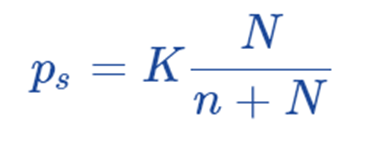
where, K = proportionality factor
For pure solvent n = 0 and the vapor pressure at this condition is P instead of Ps.
Hence, now we can write; K = P;
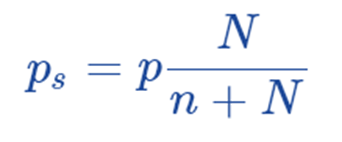
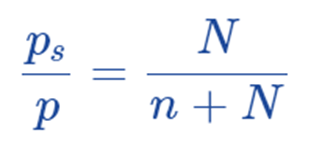
Subtracting both sides from 1, we get;
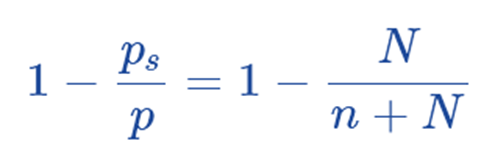
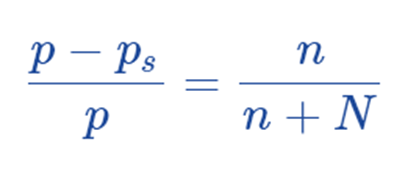
Hence, this is the raoult’s law.
Determination of Molecular Weight by Lowering of Vapour Pressure
The molecular weight of non-volatile solute can be determined by measuring the lowering of Vapor pressure experimentally. Let (w) gm of a non-volatile solute with molecular weight (m) be dissolved in (W) gm of a solvent having Molecular Weight (M).
Number of moles of solute (n) = w/m
Number of moles of solvent (N) = W/M
From the Raoult’s Law:
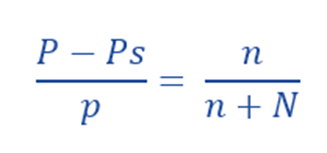
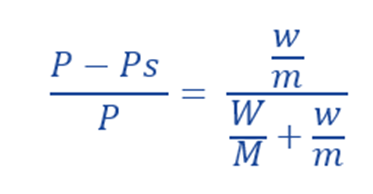
For very dilute solution, w/m is very little, ie. it is almost negligible in the denominator compared to the number of moles of the solvent. Thus,
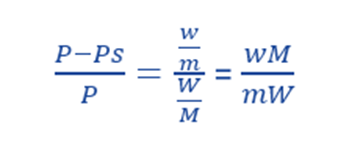
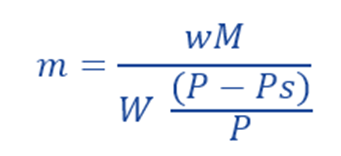
Thus, using above equation, molecular weight of a non-volatile solute can be calculated by determining the relative lowering of vapor pressure of a solution.
Methods for Determination of Lowering of vapor pressure
There are four ways for measuring vapor pressure: the Ostwald and Walker dynamic method, the equilibrium or static method, and the combination of the dynamic and manometric methods.
Static Method (Manometric Method)
A manometer is a device that monitors the pressure differences between two points. Static pressure refers to the higher reference point, while dynamic pressure refers to the lower reference point.
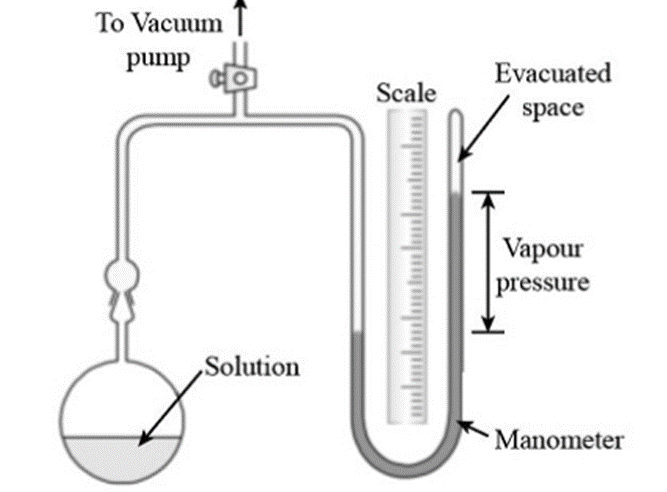
It consists of U-tube containing a liquid that is less volatile with low density (for example n-butyl phthalate).
The apparatus’s bulb is filled with the liquid or solution. A vacuum pump is then used to remove the air from the connecting tube. When the stopcock is closed, the pressure inside is only caused by the vapor that is evaporating from the solution or liquid. The pressure then can be read in manometer.
The same process is repeated by taking solution in the bulb and the pressure is read from the manometer.
The lowering of vapor pressure is then calculated using the formula:
Lowering of Vapor Pressure = P – Ps
Ostwald and Walker’s Dynamic Method
Dynamic method for measuring the lowering of vapor pressure was introduced by Ostwald and Walker. This method allows for the direct determination of the relative lowering of vapor pressure. Thus, measuring the individual vapour pressures of a solution and solvent is no longer necessary.
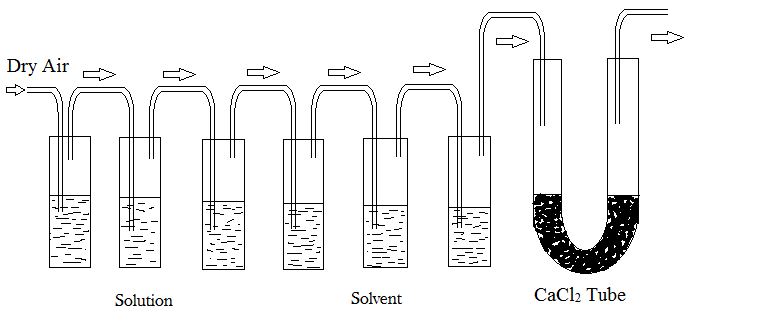
This method is based on the principle that when dry air is passed repeatedly through a series of containers containing pure solvent and solution, respectively, the air becomes saturated with the solvent vapors and there is an equal weight loss in the solution and solvent containers.
Procedure:
- The solution under investigation is contained in the first chamber while the pure solvent is contained in the second chamber. The U-tube connected at the end is filled with a measured amount of anhydrous and dry calcium chloride. There are several delivery tubes connecting the chambers to the U-tube through which dry air is passed.
- Every set is weighed independently.
- A slow stream of dry air is then pulled across the two sets of bulbs by a suction pump.
- These sets are reweighed at the conclusion of the procedure. The lowering in vapor pressure is estimated from the weight loss in each of the two sets.
- The air, solution, and solvent temperatures must remain consistent throughout.
Calculations
As the air bubbles through first chamber it is saturated up to the vapour pressure Ps of solution and then up to vapour pressure p of solvent in second chamber.
Thus the amount of solvent taken up in first chamber is proportional to Ps and the amount taken up in second chamber is proportional to (P – Ps).
If W1 and W2 be the loss of weight in first and second chamber respectively,
W1 ∝ Ps …(1)
W2 ∝ P – Ps …(2)
Adding equation (1) and (2),we have
W1 + W2 ∝ Ps + P – Ps
W1 + W2 ∝ P……(3)
Dividing (2) by (3), we can write;
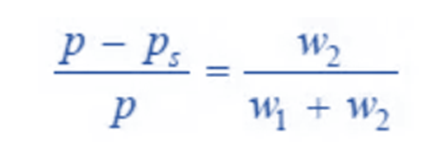
We may determine the relative lowering of vapor pressure from equation by knowing the mass loss in second chamber (W2) and the combined mass loss in the two sets (W1 + W2).
If water is the solvent, a set of calcium chloride tubes (or a series of bulbs containing concentrated H2SO4) are added to the apparatus’ end to catch the exiting water vapour. Thus, the total mass loss in two chamber is equal to (W1 + W2), which is the gain in mass of the CaCl2-tubes.
References
- https://www.vedantu.com/chemistry/colligative-properties
- Atkins, Peter and de Paula, Julio. Physical Chemistry for the Life Sciences. New York, N.Y.: W. H. Freeman Company, 2006. (124-136).
- Laidler, K.J.; Meiser, J.L. (1982). Physical Chemistry. Benjamin/Cummings. ISBN 978-0618123414
- T. Engel and P. Reid, Physical Chemistry (Pearson Benjamin Cummings 2006
- Tro, Nivaldo J. (2018). Chemistry: Structure and Properties (2nd ed.). Pearson Education. ISBN 978-0-134-52822-9.
- McQuarrie, Donald, et al. Colligative properties of Solutions” General Chemistry Mill Valley: Library of Congress, 2011. ISBN 978-1-89138-960-3.
- https://www.askiitians.com/iit-jee-solutions/ostwald-and-walker-method/
- https://www.brainkart.com/article/Raoult-s-Law-and-Dynamic-method-(or)-Ostwald—Walker-method_2764/
- https://readchemistry.com/2022/09/12/measurement-of-lowering-of-vapour-pressure/
- https://qsstudy.com/measurement-lowering-vapour-pressure-ostwald-walker-method/
- https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/vapour-pressure-of-liquid/7891/
