Magnesium is an element with the chemical symbol Mg and atomic number 12. Magnesium, which is classified as an alkaline earth metal, is solid at ambient temperature. It is a lightweight, silver-colored metal with a reflective sheen. In actuality, it is the lightest metal that exists. Magnesium is crucial for humans, animals, and vegetation. Humans and animals require it for the proper functioning of numerous enzymes and the maintenance of healthy bones. Magnesium is the fundamental molecule in chlorophyll, the substance that gives plants their green color.
Interesting Science Videos
History of Magnesium
Sir Humphry Davy was the first to isolate magnesium in 1808 by evaporating mercury from a magnesium amalgam produced by electrolyzing a mixture of moist magnesia and mercuric oxide. Magnesia is a district of Thessaly (Greece) where the mineral magnesia alba was discovered for the first time.
- In 1755, the Scottish chemist Joseph Black identified it as a distinct element. In 1618, an English farmer in Epsom endeavored to provide his cows with water from a well. They refused to consume the water due to its bitter taste. Nonetheless, the farmer observed that the water appeared to cure cuts and rashes. The popularity of Epsom salts grew. They were eventually identified as magnesium sulphate (MgSO4). In 1755, Black identified magnesium as an element.
- English chemist Humphry Davy isolated the impure metal in 1808, and French pharmacist and chemist Antoine- Alexandre Brutus Bussy isolated the purified metal in 1831. Davy electrolyzed a mixture of magnesia (magnesium oxide, MgO) and mercuric oxide (HgO) to isolate it.
- Davy’s initial suggestion for a name was magnium, but magnesium is currently in use.
Occurrence of Magnesium
Magnesium is the eighth most prevalent element in the Earth’s crust (approximately 2.5 percent) and the third most abundant structural metal after aluminum and iron.
The silvery white element, first known through compounds such as Epsom salts (the sulfate), magnesia or magnesia alba (the oxide), and magnesite (the carbonate), does not occur free in nature.
- It can be found as carbonates (magnesite, MgCO3, dolomite, CaMg(CO3)2), as well as in many common silicates such as talc, olivine, and most types of asbestos.
- It is also found in the forms of hydroxide (brucite), chloride (carnallite, KMgCl3.6H2O), and sulfate (kieserite).
- It can be found in minerals including serpentine, chrysolite, and meerschaum.
- Seawater contains approximately 0.13 percent magnesium, largely as dissolved chloride, which contributes to the bitter flavor.
Isotopes of Magnesium
Magnesium has 15 isotopes with known half-lives and mass ranges ranging from 20 to 34. Natural magnesium is a mixture of its three stable isotopes, which are present in the following percentages: 24Mg (79.0%), 25Mg (10.0%), and 26Mg (11.0%).
| Symbol | Natural Abundance |
|---|---|
| 24Mg | 78.99% |
| 25Mg | 10% |
| 26Mg | 11.01% |
24Mg Isotope:
- It is used for neutron spectrum measurement and detection of low-level neutron fluxes
- It is used for studies of isotopic effects in muonic atoms.
25Mg Isotope:
- It is used for Sodium-22 (22Na isotope) radionuclide (radioisotope) production (can be used for tracing purposes).
- 25Mg isotope is used for magnesium metabolism studies;
- It is used for nuclear physics research.
26Mg Isotope:
- This isotope is used for Aluminium-28 (28Al isotope) radionuclide (radioisotope) production (can be used in life science for healthcare and medical applications and pharmaceuticals industries).
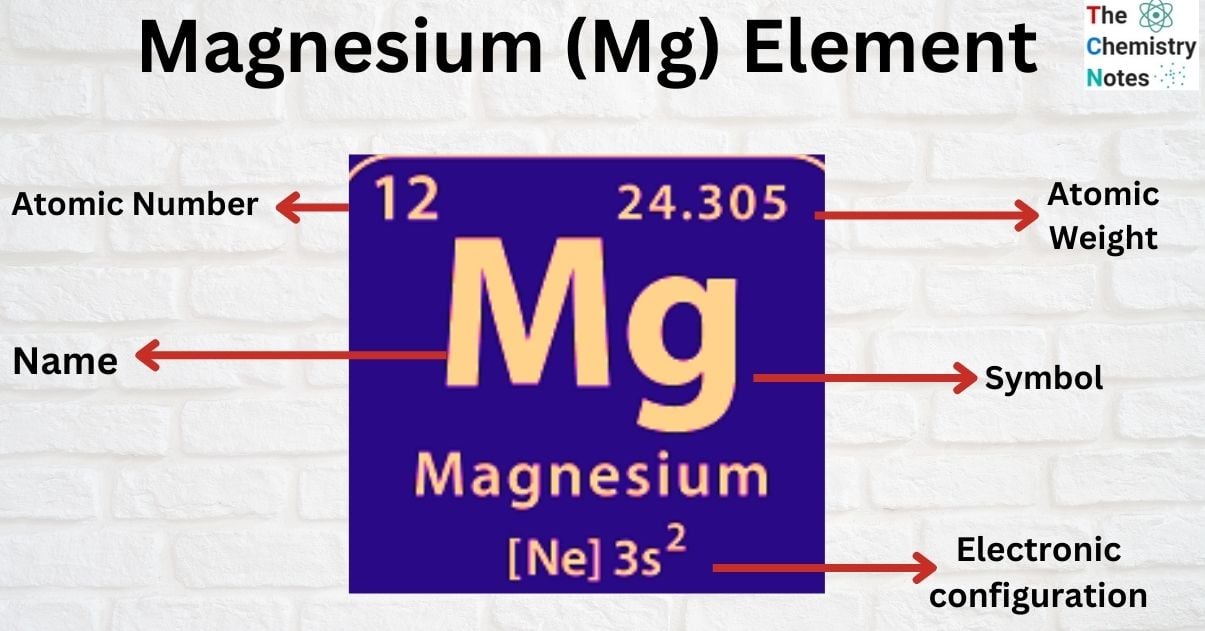
Elemental Properties of Magnesium
| Electronic Configuration | [Ne] 3s2 |
| Atomic Number | 12 |
| Atomic Weight | 24.305 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 2, 3, s-block |
| Density | 1.74 g.cm -3 at 20 °C |
| Covalent radius | 141±7 pm |
| Van der Waals radius | 0.16 nm |
| Electron shells | 2, 8, 2 |
| Electrons | 12 |
| Protons | 12 |
| Neutrons in most abundant isotope | 12 |
Physical Properties of Magnesium
Magnesium is a silvery-white, low density, relatively strong metal that tarnishes in air to develop a thin oxide covering. Magnesium and its alloys have excellent corrosion resistance and high temperature mechanical characteristics.
- Magnesium has the lowest melting point (923 K (650 °C)) and boiling point (1,363 K (1,090 °C) of any alkaline earth metal.
- Pure polycrystalline magnesium is brittle and rapidly fractures along shear bands.
- When alloyed with a small quantity of other metals, such as 1% aluminum, it becomes significantly more bendable.
- Polycrystalline magnesium’s malleability can also be considerably enhanced by lowering grain size to 1 micron or less.
| Color/physical appearance | Silver white |
| Melting point/freezing point | 650°C, 1202°F, 923 K |
| Boiling point | 1090°C, 1994°F, 1363 K |
| Density | 1.74 g/cm3 |
| Malleability | Yes |
| Ductility | Yes (low ductility) |
| Specific heat capacity | 1.02 J g-1 K-1 |
| Thermal conductivity | 156 Wm-1K-1 |
| Electrical conductivity | 22.4 x 106 Sm-1 |
Chemical Properties of Magnesium
- When exposed to air, it tarnishes slightly; but, unlike the heavier alkaline earth metals, an oxygen-free atmosphere is not required for storage because magnesium is protected by a thin layer of oxide that is quite impermeable and difficult to remove.
- At room temperature, the direct reaction of magnesium with air or oxygen produces solely the “normal” oxide MgO. This oxide, however, can be mixed with hydrogen peroxide to generate magnesium peroxide, MgO2, which can then be reacted with ozone at low temperatures to form magnesium superoxide, Mg(O2)2.
- At ambient temperature, magnesium reacts with water, however much more slowly than calcium, another group 2 metal.
- When submerged in water, hydrogen bubbles form slowly on the metal’s surface; however, when powdered, it reacts significantly faster. Higher temperatures hasten the reaction.
- Magnesium also reacts exothermically with most acids, including hydrochloric acid (HCl), creating metal chloride and hydrogen gas, as does HCl with aluminum, zinc, and many other metals.
- Magnesium is extremely combustible, especially when powdered or shaved into thin strips, but it is difficult to ignite in large quantities.
- Magnesium can also be used as an igniter for thermite, a powdered combination of aluminum and iron oxide that only ignites at extremely high temperatures.
- Organic chemistry is rife with organomagnesium compounds. They are frequently used as Grignard reagents. Grignard reagents are formed when magnesium reacts with haloalkanes. Grignard reagents include phenylmagnesium bromide and ethylmagnesium bromide.
- Beyond Grignard reagents, magnesium anthracene is a significant organomagnesium reagent, with magnesium forming a 1,4-bridge over the core ring. It is used to provide highly active magnesium. The butadiene dianion is obtained from the related butadiene-magnesium adduct.
- Magnesium also arises in organic chemistry as low valent magnesium compounds, usually as diatomic ions in the +1 oxidation state, but more recently also in the zero oxidation state or a mixture of the +1 and zero states. These chemicals are used in synthesis as reducing agents and suppliers of nucleophilic metal atoms.
- When magnesium is burned in air, it emits a brilliant-white light with strong ultraviolet wavelengths.
- In the early days of photography, magnesium powder (flash powder) was employed to illuminate the subject.
- Magnesium powder is used in fireworks and marine flares to produce a dazzling white light.
- It was also utilized in a variety of theatrical effects, including lightning, gun flashes, and supernatural apparition.
Chemical Reaction of Magnesium
- Reaction of Magnesium with Water: When magnesium is exposed to steam, it transforms from magnesium to magnesium oxide and hydrogen. When exposed to cold water, the reaction changes slightly. The reaction continues because magnesium hydroxide becomes insoluble in water.
Mg(s) + H2O(g) → MgO (s) + H2(g)
Mg(s) + 2H2O(g) → Mg(OH)2(s) + H2(g)
- Reaction of Magnesium with Oxygen: Magnesium oxide is formed when it is exposed to oxygen.
2Mg(s) + O2(g) → 2MgO(s)
- Reaction with Hydrogen: Magnesium hydride is formed when it is exposed to hydrogen.
Mg(s) + H2(g) → MgH2(s)
- Reaction with Nitrogen: Magnesium nitride is formed when it reacts with nitrogen.
3Mg(s) + N2(g) → Mg3N2(s)
- Reaction with Halogen: Magnesium is extremely reactive when reacted with a halogen. As an example, consider chloride. When magnesium(II) chloride is reacted with chloride, the result is magnesium(II) chloride.
Mg(s) + Cl2(g) → MgCl2(s)
Uses of Magnesium
- Magnesium is abundant in this green leafy vegetable. Magnesium is especially abundant in whole grains, seeds, and nuts (particularly almonds).
- Magnesium is also present in the flashbulbs. When Mg burns, it emits a brilliant light that allows photographers to snap images in low light conditions.
- Medical research employs the usage of magnesium. It is commonly used to treat skin disorders, attention deficit-hyperactivity disorder (ADHD), anxiety, mania, and recuperation after surgery, among other things.
- Magnesium is the most easily machined structural metal and is frequently utilized when a significant number of milling processes are necessary. Magnesium alloys are utilized in a variety of applications, including airplanes, spacecraft, machinery, cars, portable tools, and household products.
- Magnesium can be welded, molded, cut, and formed to meet specific needs. It is also simple to create. These structural metals are typically employed in the construction of large constructions and buildings.
- Magnesium metal can be used as a desulpherizer in iron and steel production, a deoxidizer in the thermal reduction of titanium, zirconium, and hafnium, and a nodularizer in the manufacturing of nodular cast iron.
- Magnesium is also used as an anode for cathodic protection in chemical storage tanks, pipelines, and ships, as well as in the creation of flare bombs, incendiary bombs, and pyrotechnics.
- Magnesium is the second most abundant intracellular cation and is involved in numerous metabolic processes such as glucose metabolism, ion channel translocation, stimulus-contraction coupling, stimulus secretion coupling, and peptide hormone receptor signal transduction.
Biological Role of Magnesium
- Magnesium is an essential mineral for humans and is required for the body to function properly. It is important for muscle movement, bone strength, blood sugar levels, and cardiovascular health.
- Adults contain approximately 25 grams of stored magnesium and should consume 300 to 400 mg daily. A diet rich in magnesium-rich foods, such as whole grains, nuts, seeds, and green leafy vegetables, can make it simple to meet the recommended amount.
- Magnesium is a cofactor in about 300 enzyme systems that control many biochemical activities in the body, such as protein synthesis, muscle and neuron function, blood glucose control, and blood pressure regulation.
- Energy synthesis, oxidative phosphorylation, and glycolysis all require magnesium.
- It is necessary for the production of DNA, RNA, and the antioxidant glutathione and contributes to the structural development of bone.
- Magnesium is also involved in the active transport of calcium and potassium ions across cell membranes, which is essential for nerve impulse conduction, muscular contraction, and normal heart rhythm.
Health Effects of Magnesium and Safety
An adult body has around 25 g of magnesium, with 50% to 60% of that amount found in bones and the rest in soft tissues. Blood serum contains less than 1% of total magnesium, and these levels are strictly monitored.
Normal serum magnesium values vary from 0.75 to 0.95 mmol/L.
A serum magnesium level of less than 0.75 mmol/L is characterized as hypomagnesemia. The kidney is in charge of magnesium homeostasis, excreting roughly 120 mg magnesium into the urine each day. When magnesium levels are low, urinary excretion is reduced.
There is no evidence that magnesium causes systemic poisoning, although chronic overuse of magnesium supplements and pharmaceuticals can cause muscle weakness, lethargy, and confusion.
Exposure to magnesium powder has a low toxicity and is not considered detrimental to health. Dust inhalation can irritate mucosal membranes and the upper respiratory tract. Eyes: mechanical damage or particle embedding may occur. Viewing burning magnesium powder without fire glasses may cause “Welder’s flash” due to the strong white flame. Skin: particle embedding in skin. Ingestion is unlikely; but, high amounts of magnesium powder may cause harm if consumed.
Although magnesium has not been studied, it is not thought to be carcinogenic, mutagenic, or teratogenic. Exposure to magnesium oxide fume after burning, welding, or working with molten metal can cause metal fume fever, which manifests as fever, chills, nausea, vomiting, and muscle discomfort. These often appear 4-12 hours after exposure and can last up to 48 hours. Magnesium oxide fume is a byproduct of magnesium combustion.
Dust explosions are possible when powder or granular dust is combined with air. If it is dry, it can be charged electrostatically through swirling, pneumatic transfer, pouring, and other methods.
Chemical hazards
When exposed to air or moisture, the chemical may spontaneously ignite, emitting irritating or hazardous fumes. Strong oxidants cause a violent reaction. Many compounds react violently with it, posing a fire and explosive risk. When it reacts with acids and water, it produces flammable hydrogen, posing a fire and explosion risk.
Safety
- Get some fresh air.
- Thoroughly rinse your eyes with water.
- To eliminate particles from the skin, properly wash it with soap and water.
- If a substantial amount of magnesium powder is consumed, induce vomiting and seek medical attention.
Environmental Effects of Magnesium
There is very little information available on the environmental effects of magnesium oxide fume. Other mammals who inhale magnesium oxide fume may experience comparable effects as humans.
Magnesium oxide fume registers 0.8 on a scale of 0 to 3. A score of 3 indicates a very significant environmental risk, whereas a score of 0 indicates a low risk.
The level of the material’s hazardous or poisonous character and/or its lack of toxicity, as well as the measure of its ability to remain active in the environment and whether it accumulates in living creatures, are all factors considered in determining this score. It does not account for exposure to the chemical.
Handling and Storage
Safe Handling Precautions: Avoid producing dusts. Protect yourself from physical harm. Keep away from potential ignition sources. Avoid skin and eye contact. Before eating or smoking, carefully wash your hands.
Storage Conditions, Including Any Incompatibilities: Keep in a tightly sealed container. Keep in a cool, dry place. Keep dampness at bay. Store away from oxidizers, acids, and halogens.
Magnesium Interesting Facts
- Magnetes was an ancient Greek tribe who lived in the location where magnesium carbonate was discovered and used as a laxative to treat a variety of ailments.
- When the magnesium-rich water from Epsom Spring in England was recognized to have medical powers in the 1600s, the town of Epsom quickly became a prominent health resort and spa. People even drank the salty water to ‘purify their blood’.
- Magnesium, which is found in the chlorophyll molecule of every green plant, is essential for photosynthesis, the process of absorbing energy from sunlight. In 1915, Richard Willstatter was awarded the Nobel Prize for characterizing the structure of this green plant pigment and identified magnesium as the primary constituent.
- Magnesium is formed in big stars through the fusion of helium and neon. The element is created in supernovas by combining three helium nuclei with one carbon.
- Magnesium is the eleventh most prevalent element in the human body in terms of mass. Every cell in the body contains magnesium ions.
- Magnesium is required by the body for hundreds of metabolic activities. The average person requires 250 to 350 mg of magnesium per day, or around 100 grams of magnesium per year.
- The skeleton contains around 60% of the magnesium in the human body, muscular tissue contains 39%, and extracellular magnesium contains 1%.
- Diabetes, heart disease, osteoporosis, sleep difficulties, and metabolic syndrome are all linked to low magnesium intake or absorption.
- China is the world’s top producer of magnesium, accounting for over 80% of global production.
- According to the USGS, the United States has an estimated 65 metric tons of magnesium in natural magnesite deposits. Nevada accounts for 88 percent of this total.
Here are some of the facts about this amazing element including how Magnesium has been used in medicine, and industry. Watch out this informative video.
References
- https://www.buyisotope.com/magnesium-isotopes.php
- Avedesian, M. M., and Hugh Baker. Magnesium and Magnesium Alloys. Materials Park, OH: ASM International, 1999. Print.
- Joseph Black, Experiments upon Magnesia Alba, Quick-Lime, and some other Alkaline Substances (1756)
- John Davy (Editor), The Collected Works of Sir Humphry Davy, Vol V, 1840, p110-115 Smith, Elder and Co. Cornhill.
- “Dietary Supplement Fact Sheet: Magnesium”. Office of Dietary Supplements, US National Institutes of Health. 11 February 2016. Retrieved 13 October 2016.
- https://byjus.com/chemistry/magnesium/
- Sir Humphry Davy, Elements of Chemical Philosophy., 1812, Part 1, Vol. 1, p198.
- Gay-Lussac et al, Annals of Chemistry and Physics, 1831, Vol. XLVI, p434-437.
- https://pubchem.ncbi.nlm.nih.gov/element/Magnesium#section=Sources
- Nancy E. Bernhardt, Artur M. Kasko, Nutrition for the Middle Aged and Elderly., (2008) p333. Nova Science Publishers
- Cowan, J. A. The Biological Chemistry of Magnesium. New York: VCH, 1995. Print.
- https://www.britannica.com/science/magnesium
- https://www.lenntech.com/periodic/elements/mg.htm#:~:text=Chemical%20element%2C%20metallic%2C%20symbol%20Mg,108.6%20lb%2Fft3).
- https://www.livescience.com/28862-magnesium.html
- Helmenstine, Anne Marie, Ph.D. “Interesting Facts About Magnesium.” ThoughtCo, Aug. 28, 2020, thoughtco.com/interesting-magnesium-element-facts-603362.
- https://tropeaka.com.au/blogs/the-latest/9-interesting-facts-about-the-mineral-magnesium

