The mesomeric effect, also known as resonance, is a crucial idea in organic chemistry that affects how electrons move within molecules. It’s vital for understanding how chemical compounds behave and react. This effect shows us the distribution of electrons, influencing the stability and behavior of molecules. Knowing about the mesomeric effect is key to understanding how organic chemistry reactions work.
The concept of the mesomeric effect, coupled with the notions of mesomerism and mesomer, was initially formulated by the eminent scientist Ingold in 1938. Notably, the term “mesomerism” was predominantly employed in French and German until the 1950s. However, in the English language, the term “resonance” has since become more prevalent and widely adopted. Despite the distinct nomenclature, both terms essentially encapsulate the same conceptual framework. It is noteworthy that the term “resonance” was introduced by the esteemed scientist Pauling. This overview aims to explain the mesomeric effect, its importance, and how it plays a big role in organic chemistry.
Interesting Science Videos
What is Mesomeric Effect?
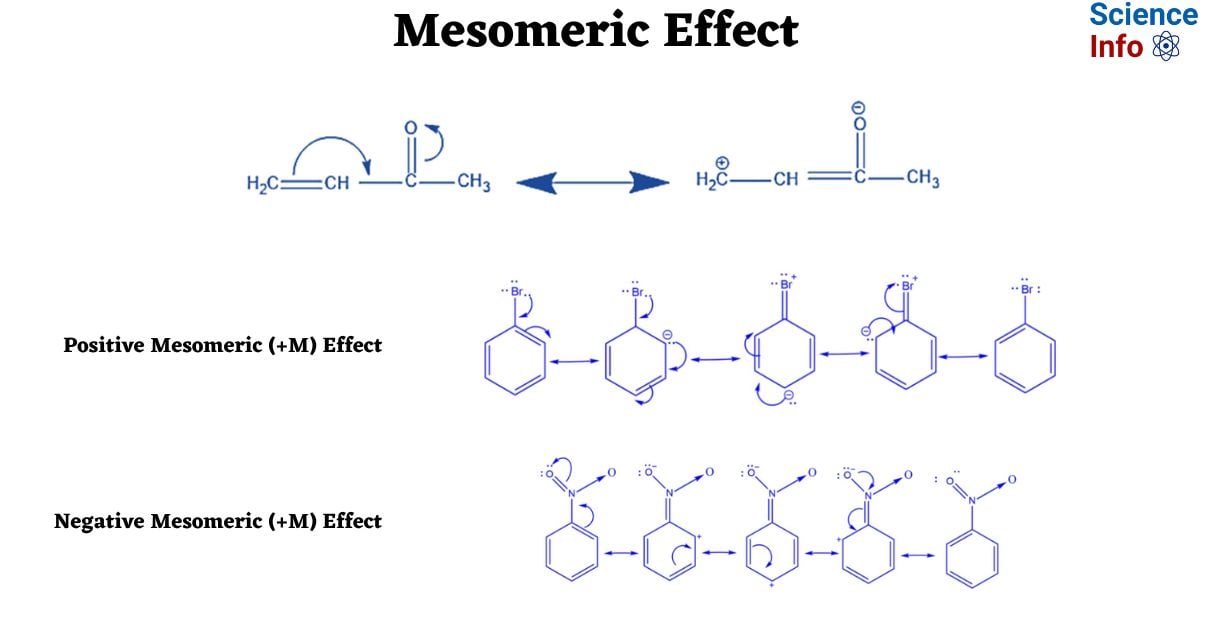
The mesomeric effect, also known as resonance effect, refers to the induction of polarity in a molecule or a conjugated system resulting from the movement of π electrons towards or away from a substituent group. Essentially, when an atom or a group of atoms donates or withdraws electrons through resonance, it is termed the mesomeric effect. This phenomenon leads to either electron-releasing or electron-withdrawing effects on substituents due to the delocalization of π− electrons.
In simpler terms, it occurs when pi electrons shift towards or away from a substituent group in a conjugated orbital system. This polarity arising from electron transfer or pi–bond electron movement defines the mesomeric effect. It can be further categorized into two types: the positive mesomeric effect (+M effect) and the negative mesomeric effect (-M effect).
The mesomeric effect, also known as resonance effect, refers to the induction of polarity in a molecule or a conjugated system resulting from the movement of π electrons towards or away from a substituent group. Essentially, when an atom or a group of atoms donates or withdraws electrons through resonance, it is termed the mesomeric effect. This phenomenon leads to either electron-releasing or electron-withdrawing effects on substituents due to the delocalization of π− electrons.
Put differently, it occurs when pi electrons shift towards or away from a substituent group in a conjugated orbital system. This polarity arising from electron transfer or pi–bond electron movement defines the mesomeric effect. It can be further categorized into two types: the positive mesomeric effect (+M effect) and the negative mesomeric effect (-M effect).
Types of Mesomeric Effect
There are two primary categories of mesomeric effects: positive mesomeric effect (+M) and negative mesomeric effect (-M). The positive mesomeric effect involves the donation of electron density by a substituent, while the negative mesomeric effect withdraws electron density.
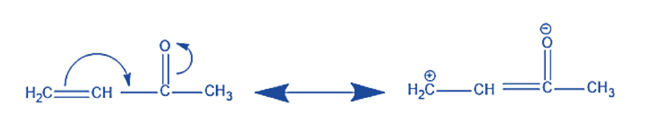
Positive Mesomeric (+M) Effect
The transfer of electron density from one functional group or atom to another nearby atom or group is known as the positive mesomeric effect, or +M effect. This donation occurs through the overlap of π orbitals or interaction of lone pairs of electrons. The result is an increase in electron density at the receiving atom or group, stabilizing positive charges and electron-deficient species. Functional groups exhibiting the +M effect include amino groups (-NH2) and hydroxyl groups (-OH).
In simpler terms, when pi electrons move towards a particular group, the electron density of the molecule or conjugated system increases, leading to the positive mesomeric effect. Examples of +M substituents are −OH, −SH, -OR, -OCOR, −NH2, -NR2, –NHCOR, Ph, –CH3, –F, –Cl, -Br, –I, etc.
The order of the +M effects is given as:
−O− > −NH2 > −OR > −NHCOR > −OCOR > −Ph > −CH3 > −F > −Cl > −Br > −I
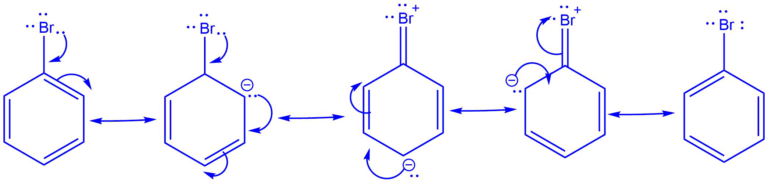
Negative Mesomeric (-M) Effect
In contrast, the negative mesomeric effect, or -M effect, involves the withdrawal of electron density from a neighboring atom or group. This withdrawal occurs when a functional group or atom possesses higher electronegativity or an electron-withdrawing capability. The result is a reduction in electron density at the receiving atom or group, stabilizing negative charges and electron-rich species. Functional groups exhibiting the -M effect include carbonyl groups (C=O) and nitro groups (-NO2).
Simply put, when pi electrons move away from the rest of the molecule towards a specific group, the electron density decreases, leading to the negative mesomeric effect. Examples of -M substituents are NO2, –CN, –SO3H, –COOH, –COOR, –CONH2, –COO−, –CHO, –COR, and so on.
The order of -M effect is given as:
−NO2 > −CN > −SO3H > −CHO > −COR > −COOCOR > −COOR > −COOH > −CONH2 > −COO−
Mechanism of Mesomeric Effect
The mesomeric effect operates through a multi-step mechanism, where the dynamic interplay of electrons within a conjugate system and substituent groups leads to a redistribution of electron density.
Electron Redistribution: The pi electrons in the conjugate system interact with electrons in the substituent group, causing a shift in electron density.
+M Effect: In the positive mesomeric effect (+M), the substituent gains electrons, increasing electron density and developing a more negative charge.
-M Effect: In the negative mesomeric effect (-M), the substituent loses electrons, reducing electron density and acquiring a more positive charge.
The change in electron density significantly influences the compound’s chemical properties, affecting its reactivity with nucleophiles or electrophiles.
The mesomeric effect originates from the overlap of p-orbitals, allowing electrons to move between atoms within a molecule. This electron movement, driven by pi electrons, either stabilizes or destabilizes the molecule based on the substituent characteristics. Understanding this mechanism is crucial for predicting how a compound will react in various organic chemistry scenarios.
Factors Affecting Mesomeric Effect
In organic chemistry, the mesomeric effect, although potent, can be influenced by various factors that are essential for understanding a molecule’s reactivity. Key considerations include:
- Steric Hindrance: When bulky substituents hinder electron movement, steric hindrance can weaken or override the mesomeric effect. This hindrance disrupts the alignment of electron orbits and necessary resonance overlap.
- Conjugation Length: The strength of the mesomeric effect is connected to the length of the conjugated system. Longer systems have a stronger effect due to better electron spread. Shortening the system or adding non-conjugated parts may weaken or eliminate the mesomeric effect.
- Electronic Effects of Neighboring Functional Groups: Nearby functional groups, whether taking or giving away electrons, change electron distribution and influence the mesomeric effect. These groups can either support or counteract the mesomeric effect, depending on their nature and position.
- Solvent Effects: The choice of solvent matters, affecting the mesomeric effect by stabilizing or destabilizing charged particles. Polar solvents strengthen the mesomeric effect, while nonpolar solvents may weaken it.
- Electronic Effects of Functional Groups: Other groups in the molecule also contribute to the mesomeric effect’s complexity. Groups that either take or give away electrons can interact with the mesomeric effect, altering electron distribution and overall electronic properties.
Understanding how these factors interact is crucial for predicting and interpreting how molecules behave in organic chemistry. While the mesomeric effect is important, considering these electronic and structural factors provides a more complete understanding of a compound’s reactivity and properties.
Applications of Mesomeric Effect
The utilization of the mesomeric effect extends across various domains within organic chemistry, offering versatile applications:
- Enhancing Carbocation Stability: The mesomeric effect plays a crucial role in stabilizing carbocations by dispersing the positive charge across multiple atoms. This dispersion makes carbocations less prone to nucleophilic attacks. An illustrative example is the enhanced stability of the benzyl carbocation compared to the tert-butyl carbocation, owing to the resonance stabilization provided by the phenyl ring.
- Aromaticity: Aromatic compounds derive heightened stability from extensive resonance stabilization. This stability arises from the delocalization of electrons over a conjugated system of atoms. Benzene, for instance, showcases high aromaticity due to the resonance stabilization of its six carbon atoms and six pi electrons.
- Influence on Acid-Base Reactions: The mesomeric effect can sway the acid-base properties of compounds. For instance, benzoic acid exhibits greater strength compared to acetic acid, thanks to the resonance stabilization of the benzoate ion.
- Stabilization of Carbanions: By reducing the electron density of carbanions, the mesomeric effect contributes to their stabilization, rendering them less susceptible to electrophilic attacks.
- Impact on Acidic and Basic Strength: The strength of acids corresponds directly to the -M effect, whereas base strength aligns with the +M effect.
- Free Radical Stability Increases: Resonance, facilitated by the mesomeric effect, enhances the stability of free radicals, making them more robust and resistant to chemical changes.
Mesomeric Effect in Organic Chemistry
Stabilization of Intermediates
The mesomeric effect plays a pivotal role in stabilizing intermediates during chemical reactions, influencing reaction pathways and product formation. Intermediates, transient species formed during reactions, often possess unpaired electrons or electron-deficient centers. The mesomeric effect occurs when adjacent atoms or groups have π bonds or lone pairs, allowing them to donate or withdraw electron density through resonance. This electron delocalization stabilizes intermediates, making them less reactive and more persistent in the reaction.
Positive Mesomeric Effect (+M): Involves the donation of electron density to stabilize positive charges or radicals in the intermediate. Examples include alkyl groups (-R), amino groups (-NH2), or hydroxyl groups (-OH).
Negative Mesomeric Effect (-M): Involves the withdrawal of electron density to stabilize negative charges in the intermediate. Examples include carbonyl groups (-C=O) or nitro groups (-NO2).
Stabilized intermediates impact reaction rates, selectivity, and overall product yield, preventing undesired side reactions and enhancing efficiency.
Impact on Acidity and Basicity
The mesomeric effect significantly influences the acidity and basicity of organic compounds, affecting their proton-donating or electron-pair donating tendencies.
Acidity: Electron-donating groups increase acidity, e.g., methoxy group (OCH3) in para-substituted benzoic acid. Electron-withdrawing groups decrease acidity, e.g., nitro group (NO2) in para-substituted benzoic acid.
Basicity: Positive Mesomeric Effect (+M): Enhances basicity by stabilizing positive charges. Example: Aniline (-NH2). Negative Mesomeric Effect (-M) decreases basicity by withdrawing electron density. Example: Amide compared to amine.
Understanding the mesomeric effect is crucial for predicting and explaining organic compound behavior in acid-base reactions.
Effects on Nucleophilic and Electrophilic Reactions
The mesomeric effect, a key player in nucleophilic and electrophilic reactions, influences reactivity and outcomes by altering electron density distribution.
Nucleophilic Reactions: Positive Mesomeric Effect (+M) increases nucleophilicity by donating electron density. Example: Grignard reagent with an ethyl group. Negative Mesomeric Effect (-M) decreases nucleophilicity by withdrawing electron density. Example: Nitro groups reducing nucleophilic reactivity.
Electrophilic Reactions: Positive Mesomeric Effect (+M)- Enhances electrophilicity by donating electron density. Example: Electrophilic aromatic substitution with alkyl groups. Negative Mesomeric Effect (-M)- Decreases electrophilicity by withdrawing electron density. Example: Carbonyl groups reducing electrophilic reactivity.
The mesomeric effect, while crucial, interacts with other factors like steric hindrance and solvent effects in determining reaction outcomes. Manipulating this effect is key to designing and controlling organic reactions.
Limitations of Mesomeric Effect
- Qualitative Nature: This effect doesn’t provide quantitative details, offering only a qualitative understanding without specifics on charge distribution within a compound.
- No Representation of Actual Movement of Electrons: Instead of depicting the actual movement of electrons, it simplifies by presenting an overview of electron distribution in a molecule.
- No Explanation on Energy Changes: The mesomeric effect lacks explanations about energy changes related to resonance structures, leaving gaps in our comprehension of the energetic aspects of the phenomenon.
- Incomplete Electronic Structure: It falls short of presenting a comprehensive electronic structure by taking an oversimplified perspective of electron distribution and ignoring certain details.
Resonance Effect Vs Mesomeric Effect
Resonance, a fundamental theory in chemistry, elucidates the interplay between lone electron pairs and bond electron pairs within a molecule. In contrast, the Mesomeric effect, another pivotal theory in chemistry, elucidates the fortification of molecules featuring diverse substituent groups and functional groups. This distinction forms the core dissimilarity between resonance and the mesomeric effect. Additionally, resonance significantly influences a molecule’s polarity directly, whereas the mesomeric effect does not exert a substantial impact in this regard. Furthermore, the causative factors for resonance involve the adjacency of double bonds to lone electron pairs, while the mesomeric effect is triggered by the presence of electron-donating or withdrawing substituent groups.
| Resonance Effect | Mesomeric Effect |
| Resonance describes how lone and bond electron pairs interact, influencing the chemical structure of a molecule. | Mesomeric effect refers to the stabilization of a molecule achieved through the presence of different functional groups or substituents. |
| Resonance occurs due to the presence of lone pairs adjacent to double bonds, leading to the delocalization of electrons. | Mesomeric effect is caused by the influence of substituents, functional groups, or conjugated systems, contributing to electron delocalization. |
| Positive resonance effect occurs when electron density increases, and negative resonance effect occurs when it decreases. | Positive mesomeric effect enhances stability, while negative mesomeric effect diminishes stability by affecting electron distribution. |
| Resonance involves electron interaction, affecting the molecule’s structure. Mesomeric effect relies on various groups impacting stability through electron delocalization. | While both contribute to stability, resonance focuses on electron pairs, and mesomeric effect emphasizes the impact of substituents and functional groups. |
Inductive Effect Vs Mesomeric Effect
| Inductive Effect | Mesomeric Effect |
| The Inductive Effect reflects a permanent polarization in sigma bonds, particularly noticeable in cases of different electronegativity between bonded atoms. It is a continuous, distance-dependent phenomenon, though relatively weaker compared to other electronic effects. | The Mesomeric Effect results from the delocalization of electrons, primarily found in unsaturated chains and conjugated systems. It represents a more extensive, permanent polarization phenomenon. |
| Electronegativity difference between bonded atoms and distance (bond length) influence the strength of the inductive effect. | The Mesomeric Effect is contingent on the substituents or functional groups within a compound, and it is notably present in molecules with at least one double bond. It is not distance-dependent but relies on the specific arrangement of atoms. |
| – Negative Inductive Effect (-I): Associated with electron-withdrawing groups. Examples include NH₃⁺, NO₂, CN, etc. -Positive Inductive Effect (+I): Linked to electron-releasing groups. Examples include C(CH₃)₃, CH(CH₃)₂, CH₂CH₃, CH₃, H. | – Positive Mesomeric Effect (+M): Notable in substituents with resonance structures that release electrons. Examples: alcohol, amine, and benzene. – Negative Mesomeric Effect (-M): Associated with substituents withdrawing electrons. Examples include acetyl (ethanoyl), nitrile, and nitro groups. |
References
- https://testbook.com/chemistry/mesomeric-effect
- https://infinitylearn.com/surge/blog/iit-jee/mesomeric-effect/
- https://chemistnotes.com/organic/mesomeric-effect/
- https://www.crackchemistry.in/mesomeric-effect-definitiontypesorder-and-uses/
- https://byjus.com/jee/mesomeric-effect/
- https://unacademy.com/content/jee/study-material/chemistry/mesomeric-effect/
- https://www.geeksforgeeks.org/mesomeric-effect/
- https://www.geeksforgeeks.org/mesomeric-effect/
- https://unacademy.com/content/jee/study-material/chemistry/types-of-mesomeric-effects/
- https://www.chemistrywithdrsantosh.com/2023/07/mesomeric-effect-understanding-its-significance-in-organic-chemistry.html
