The molarity is described as the total number of moles of solute contained in a particular solution per liter. It is also known as “molar concentration” and is pronounced “molar.” It is denoted by the letter “M,” and its unit is mol/L.
The molarity of a particular solution depends on the physical characteristics of the system, such as pressure and temperature, unlike mass, which fluctuates with alterations in the system’s physical parameters. The volume of the majority of solutions has a little temperature dependence owing to thermal expansion, which is one disadvantage of molarity. As a result, molarity is not frequently utilized in thermodynamics.
Interesting Science Videos
Molarity Formula
The formula for determining the molarity of a solution is as below:
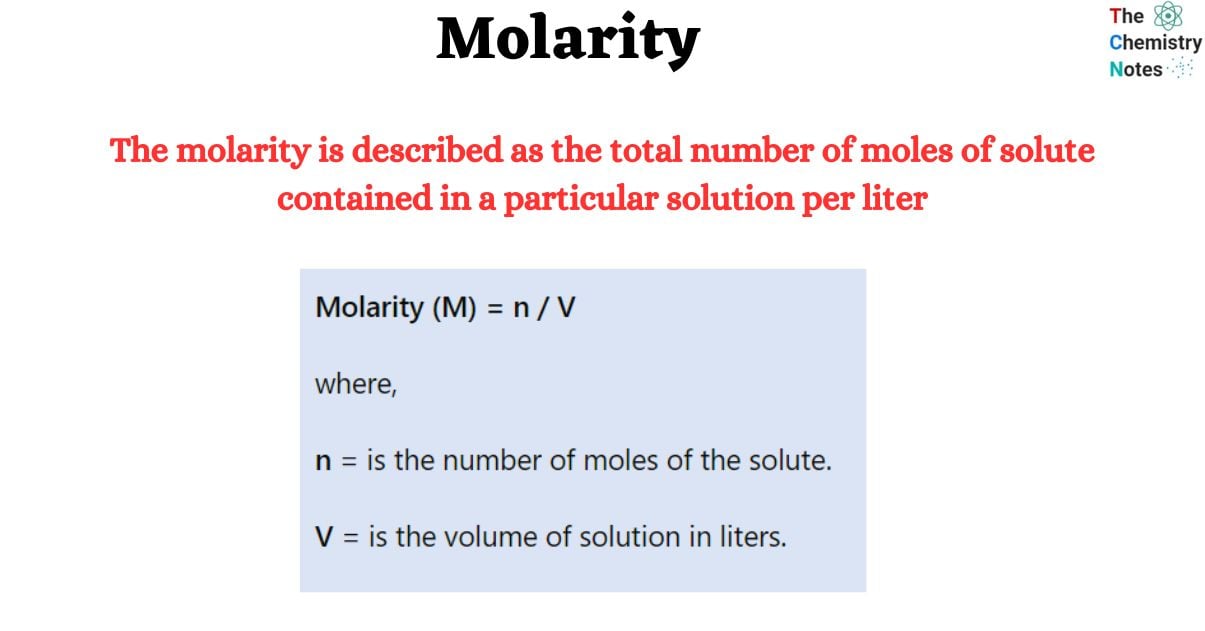
Molarity (M) = n / V
where,
n = is the number of moles of the solute.
V = is the volume of solution in liters.
Formula to calculate the Number of Moles of Solute
(n) = Mass of the solute / Molar mass of the soluteMolarity Unit
- The letter “M” is the symbol for molarity.
- Mole/liter is the metric measurement for molarity.
Molarity Equation
To make a concentrated solution, it must first be diluted with the solvent. As a result, the solution’s volume changes. The following equation is used to calculate the volume of an unknown solution from a known molarity solution:
M1V1 = M2V2
Where,
M1 = the initial molarity of the given solution.
M2 = is the molarity of the new solution.
V1 = is the initial volume of the given solution, and
V2 = the volume of the new solution.
Calculating Molarity
To determine a solution’s molarity, follow the instructions below:
Step 1: Determine how many moles of the solute are in the solution.
Step 2: Measure the volume of solution in liters.
Step 3: Use the equation to determine the solution’s molarity.
Molarity = Number of Moles of Solute / Volume of Solution in litersStep 4: Replacing all the variables in the aforementioned formula will provide the solution’s molarity.
Advantages of Molarity
There are some advantages of using molarity which are mentioned below:
- The solute can be measured in grams, converted to moles, and combined with a volume, making it simple and practical to use.
- The total molar concentration is the sum of all the molar concentrations. This enables density and ionic strength calculations.
Limitation of Molarity
- Temperature has an impact on molarity. This is because a liquid’s volume is influenced by temperature. This is not an issue if all measurements are carried out at the same temperature, such as room temperature. However, it’s best practice to mention the temperature when mentioning a molarity figure. When creating a solution, keep in mind that if you use a hot or cold solvent but store the finished product at a different temperature, the molarity will alter significantly.
Video Reference
References
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/16%3A_Solutions/16.08%3A_Molarity
- https://www.geeksforgeeks.org/molarity/
- https://www.khanacademy.org/science/chemistry/states-of-matter-and-intermolecular-forces/mixtures-and-solutions/a/molarity
- https://byjus.com/molarity-formula/
- https://www.thoughtco.com/molarity-definition-in-chemistry-606376
- https://www.mlsu.ac.in/econtents/1844_SPOT%20I.pdf

