Moscovium is a synthetic transition metal with an atomic number of 115 and is represented by the symbol ‘Mc’ in the periodic table. It is silvery in appearance and belongs to the p-block of period 7 of the periodic table. Only tiny quantities of Moscovium have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Moscovium is an extremely radioactive element that does not occur naturally, is produced inside a laboratory setting, and decays within milliseconds after being synthesized. This element, formerly known as ununpentium or eka-bismuth, was created in 2003 by a group of Russian and American scientists working at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. The team was led by Russian physicist Yuri Oganessian. Moscovium was named after the Russian region of Moscow, where the Joint Institute for Nuclear Research (JINR) is located. Moscovium was formally added to the periodic table on November 28th, 2016.
Interesting Science Videos
History and Discovery of Moscovium
- Moscovium was known as eka-bismuth or element-115 under Mendeleev’s taxonomy of unknown elements.
- Moscovium was created by an American-Russian team led by Yuri Oganessia in Dubna, Russia, in 2003.
- JINR collaborated with Lawrence Livermore National Laboratory to successfully develop element 115.
- The experiment was initiated on July 14, 2003, and concluded on August 10, 2003.
- A cyclotron (a particle accelerator) was used to create a stream of calcium ions which were blasted at an americium target layer placed on titanium foil.
- Four atoms of element 115 (Moscovium) were formed, which alpha decayed to produce element 113 (Nihonium).
- They attacked americium-243 with calcium-48 nuclei, resulting in four moscovium-287 atoms.
- Moscovium-289 and moscovium-290 are two of the heavier isotopes of moscovium that were identified in 2010.
- The name Moscovium was chosen in honor of the Moscow Oblast, where JINR is based.
- Moscovium was formally added to the periodic table on November 28th, 2016.
Occurrence of Moscovium
- Moscovium can be synthesized artificially. It’s a synthetic element that is extremely unstable. Its half-life is only a few seconds.
- Mc is a synthetic radioactive metal formed by nuclear bombardment and has only been manufactured in trace amounts. And it is only found in specialized laboratories due to its rapid decay.
- The first atoms were formed by bombarding americium-243 with calcium-48 ions, resulting in four Moscovium atom (Mc-288 plus three neutrons, which decayed into Nh-284, and Mc-287 plus four neutrons, which further decayed into Nh-283).
- Since just about four atoms of element 115 have ever been created (by nuclear processes involving the fusion of calcium nuclei with americium nuclei), extraction of a detectable quantity has never been possible, and may never be.
- Also with the decay of the first few moscovium atoms, nihonium was detected.
- There are four isotopes of moscovium, ranging in mass from 287 to 290.
Elemental Properties of Moscovium
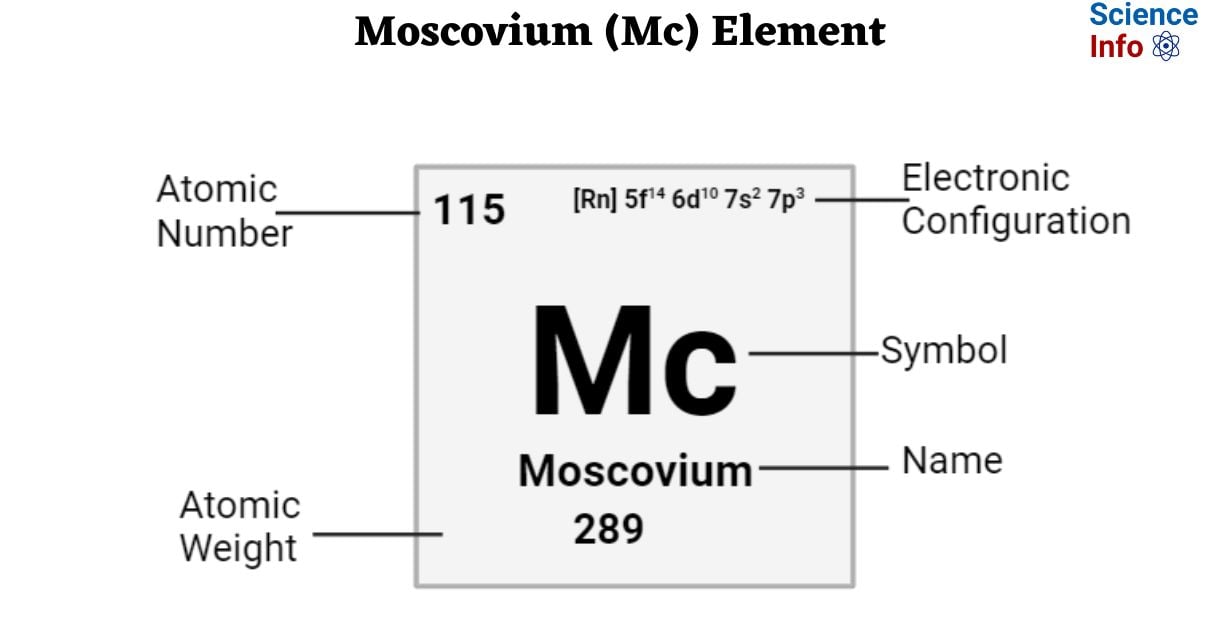
| Electronic Configuration | [Rn] 5f14 6d10 7s2 7p3 |
| Atomic Number | 115 |
| Atomic Weight | 289 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Transactinide, 7, p-block |
| Density | 13.5 g/cm3 (estimated) |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 18, 5 (estimated) |
| Electrons | 115 |
| Protons | 115 |
| Neutrons | 174 |
Isotopic Information of Moscovium
- Moscovium has no stable isotopes naturally, but they can be created in a laboratory setting.
- All of the Mc isotope are unstable and radioactive.
- All isotopes of Mc decay via alpha decay or spontaneous fission, but none of them undergo beta decay.
- Moscovium has four known isotopes but two of the heaviest isotopes are: 289Mc and 290Mc.
- Moscovium-290 has a half-life of about 0.8 seconds and is currently the most stable isotope of moscovium known to scientists.
- Moscovium is on the edge of the island of stability.
- It is projected that Moscovium-291 will have a long half-life of several seconds.
- Moscovium-289 and 290 are formed as daughter isotopes of tennessine isotopes (293 and 294).
- Moscovium atoms decay by emitting alpha particles into Nihonium in less than 100 milliseconds.
Physical Properties of Moscovium
- Moscovium’s instability makes it difficult to conduct an objective examination of its physical properties.
- Given its swift disintegration, only a few properties of Moscovium have been studied to date.
- Moscovium is a synthetic, super-heavy transactinide element.
- It is found in the 7th period, the 15th Group (pnictogens), and the p-block of the periodic table.
- Though it hasn’t been proven to function as a heavier homologue of the pnictogen bismuth, it is categorized as the heaviest pnictogen in group 15.
- Moscovium is expected to have some features in common with its lighter homologues, nitrogen, phosphorus, arsenic, antimony, and bismuth, as well as to be a post-transition metal, but it is expected to contrast considerably from them.
- Moscovium is expected to have a melting point of about 400°C, which is comparable to nihonium.
- As with nihonium, the boiling point of moscovium is expected to be approximately 1100°C.
- The atomic mass of moscovium is anticipated to be 289. (The atomic mass of man-made trans-uranium elements is calculated using the periodic table’s longest-lived isotope. These atomic weights should be considered tentative because a new isotope with a longer half-life may be created in the future).
- A density of around 13.5 g/cm3 is anticipated for moscovium.
- Moscovium shares a metallic binding strength with nihonium.
- The isotope of moscovium with the longest half-life is 0.8 seconds.
Chemical Properties of Moscovium
- Moscovium is a highly radioactive element. Its chemical properties have yet to be completely investigated. Isotopes have short half-lives, and the compounds they contain are highly volatile, making conclusive chemical analysis challenging.
- There have been no experimental measurements of moscovium compounds, and all known predictions are theoretical.
- It is the heaviest element in the 15th group of the periodic table.
- Given that both have a single, loosely bound electron outside of a quasi-closed shell, moscovium and thallium should share many properties.
- Moscovium is expected to act similarly to other pnictogens in the absence of experimental data. It might mostly resemble bismuth.
- Moscovium compounds are likely to prefer the ‘+1’ oxidation state.
- Moscovium compounds are not expected to have an oxidation state of ‘+5’, as such compounds are thought to be unattainable in nature.
Chemical Reaction of Moscovium
The reactions of moscovium are not conclusive.
- Reaction of Moscovium with Air
Only a few atoms of moscovium have ever been created, hence its reactivity with air is unknown. One would expect its behavior to be comparable to that of bismuth (directly above moscovium on the periodic table) and antimony (two places above).
- Reaction of Moscovium with Water
Only a few atoms of moscovium have ever been created, therefore its reactivity with water is undetermined. One would expect its behavior to be comparable to that of bismuth (directly above moscovium on the periodic table) and antimony (two places above).
- Reaction of Moscovium with Halogens
Since hardly a few atoms of moscovium have ever been created, its reactivity with the halogens is unknown. Its behavior is expected to be comparable to that of bismuth (directly above moscovium in the periodic table) and antimony (two places above).
- Reaction of Moscovium with Acids
Given that just some atoms of moscovium have ever been created, its reaction with acids is unclear. Its behavior is expected to be comparable to that of bismuth (directly above moscovium in the periodic table) and antimony (two places above).
Synthesis of Moscovium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- Americum-243 is bombarded with calcium-48, resulting in moscovium-287 and moscovium-288.
Uses of Moscovium
- Given that few atoms of this metal have been synthesized thus far, there are currently no specific or specialized applications for moscovium other than scientific research.
- A constant experimental study aimed at achieving an obvious conclusion requires a large number of atoms. Perhaps a few moscovium atoms have been produced thus far.
Health Effects of Moscovium
- Moscovium is a highly unstable chemical; when created, it swiftly decomposes into other elements, therefore it has no impact on human health. However, being a radioactive element it must be toxic.
Environmental Effects of Moscovium
- Moscovium’s environmental effects are negligible due to its short half-life (just a few seconds).
Video Reference
References
- https://periodic-table.com/moscovium/
- https://www.webelements.com/moscovium/
- https://www.thoughtco.com/moscovium-facts-element-115-4122577
- https://chemicalengineeringworld.com/moscovium-element-properties-and-information/

