Neon (atomic number 10, symbol Ne) is an inert noble gas and a chemical element. The name neon derives from the Greek word νέoν, the neuter singular form of νέος (neos), meaning ‘new’. Neon is chemically inert, and there are no known uncharged neon compounds. This element has the narrowest liquid range of all the elements and is the second-lightest noble gas. It has three times the cooling capacity of liquid hydrogen and forty times the cooling capacity of liquid helium. In terms of metallicity, neon is a non-metallic element in the modern periodic table. The outermost shell of neon is totally filled, setting it apart from the other elements on the periodic table.
Interesting Science Videos
History of Neon
Neon was discovered in 1898 by British scientists W. Ramsay and M. W. Travers, who collected its components from condensed air as it evaporated.
- The noble gases krypton and xenon were discovered in the same set of tests (helium and argon had been discovered earlier). That was a tremendous accomplishment, given the rarity of these gases and the chemistry lab technology of the period.
- Almost immediately, Ramsay and Travers discovered that Ne glows brilliantly red-orange when treated to electric discharge in a vacuum. G. Claude at Air Liquide (Paris) started making and selling neon discharge tubes for use in billboards and other advertising displays in 1912.
- Over time, they became increasingly commonplace in holiday design. Ne alone emits a red light; other colors come from other noble gases.
- Ramsay’s son gave neon its name. The name neon is derived from the Greek word “novum,” which means “new.”
Occurrence of Neon
While neon is the fifth most prevalent element in the universe, it is extremely rare on Earth. Just 0.0018% of neon is found in the Earth’s atmosphere.
- Ne makes up one part in 55,000 of the Earth’s atmosphere, or 18.2 ppm by volume (about the same as the molecule or mole fraction), or one part in 79,000 by mass.
- Ne is more commonly found in stars and the sun. The amount of Ne in the cosmos is around one part in 750; in the Sun and, probably, in the proto-solar system nebula, it is approximately one part in 600.
- There are traces of Ne in volcanoes, vents, and diamonds.
- Nonetheless, it is produced commercially by fractional distillation of liquid air.
Isotopes of Neon
The isotopes of neon are 20Ne (90.48%), 21Ne (0.27%), and 22Ne (9.25%).
- 21Ne and 22Ne are both primordial and nucleogenic (created by nuclear reactions of other nuclides with neutrons or other particles in the environment), and their natural abundance changes are well characterized.
- In contrast, 20Ne (the predominant primordial isotope produced by stellar nucleosynthesis) is not known to be nucleogenic or radiogenic, with the exception of the decay of oxygen-20, which is produced in extremely uncommon cases of cluster decay by thorium-228. The causes of its variation on Earth are the subject of heated debate.
- The strange primordial rare gas components in the Earth, presumably representing solar neon, were attributed to the 20Ne-enriched components.
- High 20Ne abundances have also been discovered in diamonds.
Elemental Properties of Neon
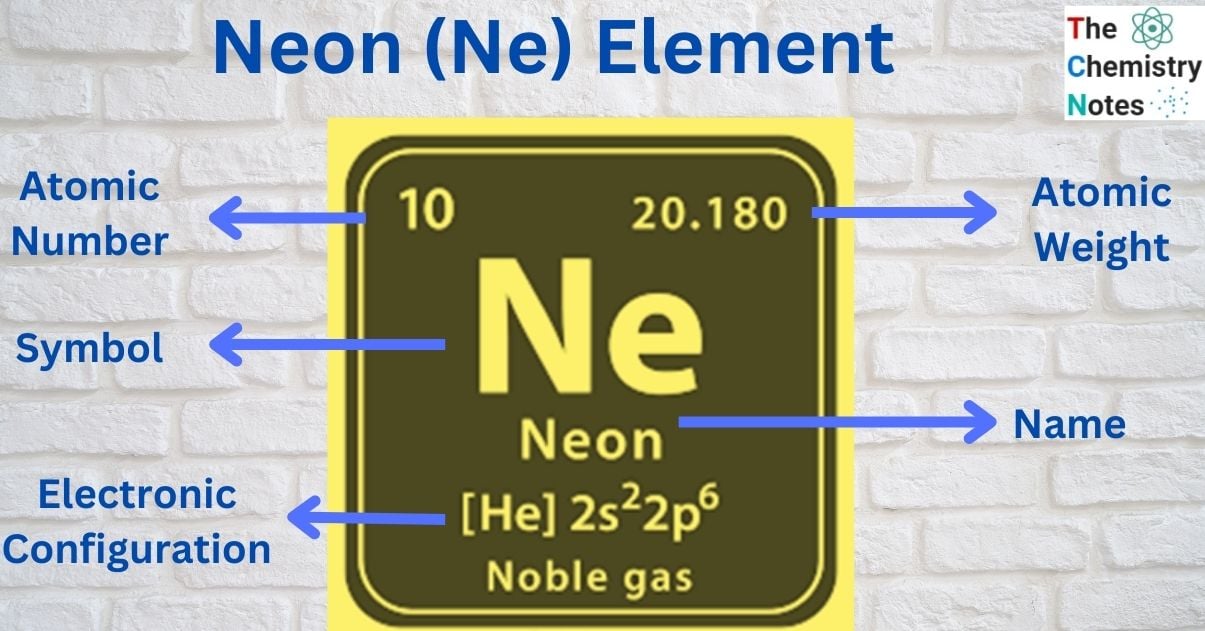
| Electronic Configuration | 1s2 2s2 2p6 |
| Atomic Number | 10 |
| Atomic Weight | 20.1797 g.mol -1 |
| State at 20°C | gas |
| Group, Period, and Block | 18, 2, p-block |
| Density | 0.000825 g cm-1 |
| Covalent radius | 48 pm |
| Van der Waals radius | 0.16 nm |
| Electron shells | 2,8 |
| Electrons | 10 |
| Protons | 10 |
| Neutrons in most abundant isotope | 10 |
Physical Properties of Neon
- Neon is a colorless, odorless, and tasteless noble gas with the symbol Ne and atomic number 10 that is present in tiny amounts in the Earth’s atmosphere.
- It is distinguished by its remarkable capacity to create a characteristic reddish-orange glow, as a consequence of which it is commonly utilized in the creation of advertising signs.
- It is the second lightest noble gas in the group, with only helium being lighter.
- At room temperature, Ne exists in the form of a gas.
- Because it has a density of 0.9002 g/L, a 1 liter container of Ne gas will weigh less than 1 gram.
- The melting point of Ne is 24.56 K (-415.46° F), whereas the boiling point is 27.07 K (-410.94 °F).
- The refrigerating capacity is 40 times greater than that of liquid helium, which explains its use as a cryogenic refrigerant.
| Melting Point | −248.59°C, −415.46°F, 24.56 K |
| Boiling Point | −246.046°C, −410.883°F, 27.104 K |
| Density | 0.000825 g cm-1 |
| Ionization Energies | 1st: 2080 kJ.mol -1 2nd: 3952 kJ.mol -1 |
| Heat of Vaporization | 1.71 kJ/mol |
| Heat of Fusion | 0.335 kJ/mol |
| Molar Heat Capacity | 24.440 J/(mol·K) |
| Standard Electrode Potential | 6122 kJ.mol -1 |
Chemical Properties of Neon
Neon is a chemically inactive element. Thus yet, no other element or chemical has been able to react with Ne. The most stable or inert atoms have 8 electrons in their outer shell. Because of their exceedingly low chemical reactivity, they are referred to as inert gases.
Uses and Applications of Neon
- The most common application of Ne is in neon signs and advertising boards. In the discharge tube, it glows reddish-orange. Additional colors are created by covering glass with various colored phosphors.
- Ne is used in indicators, television tubes, lightning arrestors, and other applications.
- It is utilized in the commercial refrigeration industry as a cryogenic refrigerant. It outperforms helium by 40 times and liquid hydrogen by three times.
- A gas laser is created by combining Ne and helium.
- In several types of gas-filled electron tubes, Ne is used solo or in mixes with other gases.
- Because helium is less soluble in blood than nitrogen at high pressure, marine divers utilize a combination of helium and Ne for breathing.
Health Effects of Neon
When this liquid escapes its container, it evaporates fast, supersaturating the air and posing a substantial suffocating danger in small spaces. Consequences of exposure are:
- A simple asphyxiant.
- Frostbite occurs when skin comes into touch with fluids.
- Frostbite occurs in the eyes when they come into touch with fluids.
- Excessive concentrations can cause dizziness, nausea, vomiting, loss of consciousness, and death. Death can occur as a result of mistakes in judgment, disorientation, or loss of consciousness that prohibit self-rescue. When oxygen levels are low, unconsciousness and death can happen in seconds.
- The impact of simple asphyxiant gases is proportional to the amount (partial pressure) of oxygen in the air that is inhaled. Before noticeable symptoms appear, the oxygen concentration in the air may be reduced to 75% of its typical level. This, in turn, necessitates the presence of a simple asphyxiant in a concentration of 33% in the air-gas mixture. When the simple asphyxiant concentration exceeds 50%, significant symptoms might occur. A 75% concentration is lethal in minutes.
- Rapid respirations and air hunger are the initial indications of a simple asphyxiant. Mental awareness is reduced, and muscle coordination is hampered. Subsequently, judgment becomes impaired, and all feelings are dampened. Emotional instability is frequently the outcome, and weariness sets in quickly.
- When the asphyxia worsens, there may be nausea and vomiting, prostration and loss of consciousness, and lastly convulsions, a profound coma, and death.
Neon: 10 Incredible Facts
- Neon has the most limited liquid range of any element. The element occurs in liquid form at just 2.5°C.
- It is the second-lightest noble gas, behind helium.
- While being very scarce on Earth, Ne is the fourth-most plentiful element in the cosmos. It accounts for 18 parts per million of air.
- The most well-known application of the element is the neon sign.
- The density of Ne is around two-thirds that of air. As a result, a neon balloon will float, but it will ascend at a slower rate than a helium balloon since helium is lighter.
- Breathing Ne will raise the pitch of your voice, although not nearly as much as helium will.
- The name neon is derived from the Greek word “neos,” which means “new.”
- Liquid Ne is used as a cryogenic refrigerant and to freeze corpses (eerie!).
- He and Ne are the two elements that do not form compounds with other elements, despite the formation of ions and ligands.
- Ne has three naturally occurring stable isotopes and 17 radioactive isotopes.
ATTENTION! This video shows dangerous experiments! Do not repeat the experiments shown in this VIDEO!
References
- https://www.acs.org/molecule-of-the-week/archive/n/neon.html
- Naturally occurring isotope abundances: Commission on Atomic Weights and Isotopic Abundances report for the International Union of Pure and Applied Chemistry in Isotopic Compositions of the Elements 1989, Pure and Applied Chemistry, 1998, 70, 217
- Black, D.C. (1972).” On the origins of trapped helium, neon and argon isotopic variations in meteorites, II. Carbonaceous chondrites.” Geochim. et Cosmochim
- https://www.lenntech.com/periodic/elements/ne.html
- https://www.britannica.com/science/neon-chemical-element
- https://chemistrytalk.org/neon-element/

