Nihonium is a synthetic transition metal with an atomic number of 113 and is represented by the symbol ‘Nh’ in the periodic table. It is silvery in appearance and belongs to the p-block of period 7 of the periodic table. Only tiny quantities of Nihonium have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Nihonium, an extremely radioactive element that does not occur naturally, and is produced inside a laboratory setting and decays within milliseconds after being synthesized. Nihonium was identified on August 12, 2012, at Kosuke Morita’s RIKEN Nishina Center for Accelerator-Based Science in Wakō, Japan. It was the first chemical element found in Asia. The name for the element “Nihonium” is derived from the Japanese word “Nihon,” which refers to Japan, where it had been identified initially.
Interesting Science Videos
History and Discovery of Nihonium
- In 1999, the Joint Institute of Nuclear Research (JINR) reported that it discovered a decay chain that, when combined into a hot-fusion procedure, generates isotope-290 of element 113. Several research organizations attempted to replicate the chain, but all of them failed, and the outcome was never verified.
- RIKEN, Japan’s Institute of Physical and Chemical Research, is credited with discovering element-113, Nihonium.
- The very first Nihonium isotope to be created is 284Nh. On August 12, 2012, a team led by Kosuke Morita from the RIKEN Nishina Center for Accelerator-Based Science in Wakō, Japan, determined that the element 113 isotope is a decay product of the 288Mc.
- The researchers employed RIKEN’s Linear Accelerator Facility and the GARIS ion separator at Wako, Japan, to investigate cold fusion processes and the moscovium’s radioactive decomposition.
- Teams of scientists from the US, Germany, Sweden, China, Russia, and Japan were part of the process of confirming the assertions made by the two groups of experts concerning the evidence.
- The term ‘Nihonium’ (from ‘Nihon’, the Japanese word for Japan) was given by a team of discoverers who wanted to pay honor to their home country.
- The International Union of Pure and Applied Chemistry confirmed the name “Nihonium” for the newly discovered element 113.
Occurrence of Nihonium
- Nihonium can be synthesized artificially. It’s a synthetic element that is extremely unstable. Its half-life is only a few seconds.
- Nihonium is a synthetic radioactive metal formed by nuclear bombardment and has only been manufactured in trace amounts.
- The element was generated in a cold fusion process involving a bismuth-209 target with zinc-70 ions.
- Nihonium has eight isotopes detected so far; however only one isotope with a known half-life: 278Nh.
Elemental Properties of Nihonium
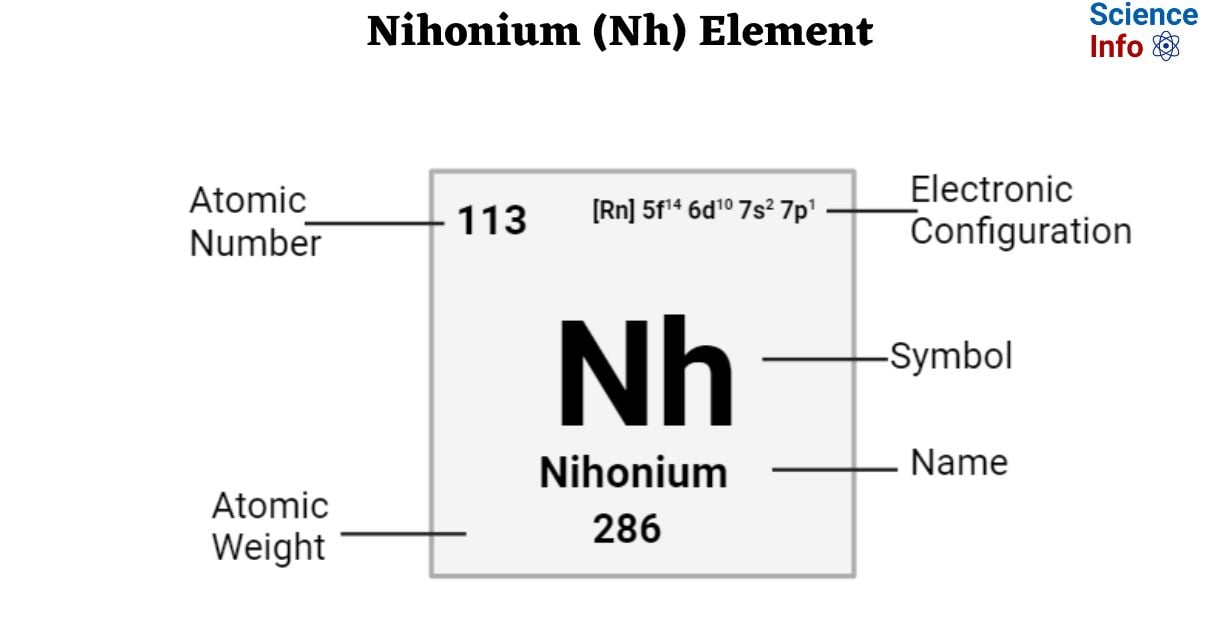
| Electronic Configuration | [Rn] 5f14 6d10 7s2 7p1 |
| Atomic Number | 113 |
| Atomic Weight | 286 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Trans-actinides, 7, p-block |
| Density | 16 g/cm3 (estimated) |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 18, 3 (estimated) |
| Electrons | 113 |
| Protons | 113 |
| Neutrons | 173 |
Isotopic Information of Nihonium
- Nihonium has no stable isotopes naturally, but they can be created in a laboratory setting.
- All of the Nihonium isotopes are unstable and radioactive.
- All isotopes of Nihonium decay via alpha decay or spontaneous fission, but none of them undergo beta decay.
- The atomic mass of Nihonium isotopes are 278Nh, 282Nh, 283Nh, 284Nh, 285Nh, 286Nh, 287Nh, and 290Nh.
- Isotopes are created when a heavier element decays or when two light nuclei fuse.
- The heavier isotopes are more stable than the lighter ones.
- Most Nihonium isotopes decay by emitting alpha particles into roentgenium isotopes.
- 286Nh has a half-life of around 8 seconds, whereas nihonium-285 has an anticipated half-life of about one second.
- Recent data reveals that 284Nh decays through electron capture, producing copernicium-284.
Physical Properties of Nihonium
- Nihonium’s volatility makes it difficult to perform a statistically significant study of its physical properties.
- Due to its rapid disintegration, only few properties of Nihonium have been investigated until now.
- Nihonium is a synthetic, super-heavy transactinide element.
- It is found in the 7th period, the 13th Group, and the p-block of the periodic table.
- Nihonium belonging to the boron group (13 groups) of the periodic table, its properties are considered to be similar to thallium. It’s claimed to be denser than thallium.
- The melting point and the boiling point of the element 113 is yet to be known.
- The atomic mass of Nihonium is 286. (The atomic mass of man-made trans-uranium elements is calculated using the periodic table’s longest-lived isotope. These atomic weights should be considered tentative because a new isotope with a longer half-life may be created in the future).
- Nihonium is projected to have a hexagonal, tightly packed structure.
- The longest-lived Nihonium isotope has a half-life of approximately 8 seconds.
Chemical Properties of Nihonium
- Nihonium is an extremely radioactive element. It’s chemical characteristics have yet to be thoroughly researched. Isotopes have short half-lives, and the molecules they contain are extremely volatile, making statistically meaningful chemical analysis difficult.
- There have been no experimental measurements of Nihonium compounds, and all known predictions are theoretical.
- The heaviest element in the 13th group of the periodic table, Nihonium is a transactinide and the first member of the p-series.
- It is located under thallium, gallium, indium, boron, and aluminum.
- Nihonium is expected to be chemically distinct from thallium and other members of group 13, possibly due to the spin-orbit splitting of the 7p shell.
- It has been suggested that Nihonium is likely to be more stable in the oxidation state +1 (as are the halogens fluorine, chlorine, bromine, iodine, and astatine) than in the oxidation state +3.
- Based on characteristic studies, the probability of Nihonium adopting three other oxidation states is identified: +5, +3, and +2.
- Nihonium’s adsorption ability on gold surfaces has been hypothesized to be similar to astatine.
Synthesis of Nihonium
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- The bombardment of bismuth-209 with zinc-70 produces nihonium-278.
Uses of Nihonium
- Given that few atoms of this metal have been produced so far, there are currently no particular or specialized applications for Nihonium outside of scientific research.
- Furthermore, because it is unavailable in nature, Nihonium is used only by scientific researchers, with no reported negative consequences or uses for the metal among individuals and organizations.
- A consistent scientific experiment aiming to produce an obvious result necessitates a large number of atoms of the same element. Only a few Nihonium atom have been yielded thus far.
Health Effects of Nihonium
- Nihonium is a very unstable chemical; when created, it swiftly decomposes into other elements, therefore it has no impact on human health.
Environmental Effects of Nihonium
- Nihonium’s environmental effects are negligible due to its short half-life (just a few seconds).
Video Reference
Reference
- https://periodic-table.com/nihonium/
- https://www.chemicool.com/elements/nihonium.html
- https://www.vedantu.com/chemistry/nihonium
- https://chemicalengineeringworld.com/nihonium-element-properties-and-information/
- https://www.thoughtco.com/ununtrium-facts-element-113-606492
- https://thechemicalelements.com/nihonium/

