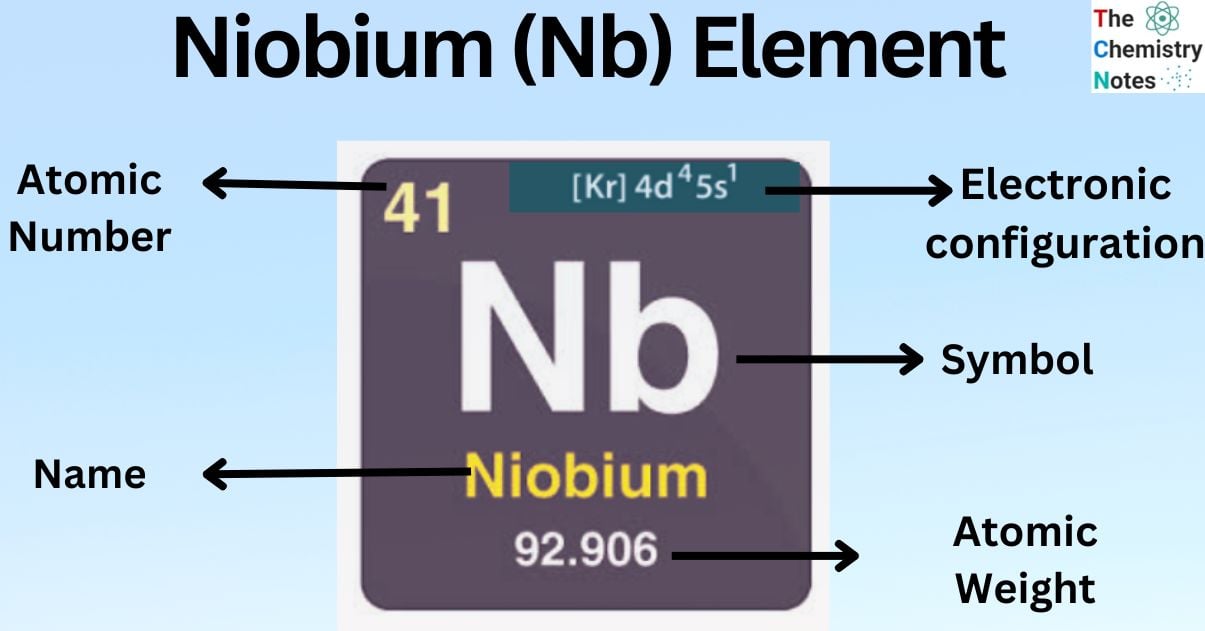
Niobium is a metallic element with the atomic number 41 and is represented by the symbol ‘Nb’ in the periodic table. It belongs to the d-block of group 5 of the periodic table. It is a rare soft metal with a grayish metallic appearance.
Nb makes up the 0.002 percent of Earth’s crust. It is the 34th most prevalent element in the Earth’s crust. Niobium does not occur freely in the nature. It is mostly found in minerals which include columbite (Fe, Mn)Nb2O6 and columbite-tantalite (or coltan, (Fe, Mn)(Ta, Nb)2O6) among others.
Interesting Science Videos
History of Niobium
- In 1734, John Winthrop Younger, the first governor of Connecticut, noticed a new mineral. He named the mineral columbite (Fe, Mn, Mg)(Nb, Ta)2O6 after himself and sent a sample to London’s British Museum.
- For several decades, the columbite stayed among the museum’s mineral collections, eventually being studied by Charles Hatchett, an English chemist, in 1801. Hatchett detected an unknown element in the columbite but had no way to extract it. He gave the new element the name columbium.
- When English chemist William Hyde Wollaston investigated the minerals columbite and tantalite in 1809, he concluded that columbium was in fact the element tantalum. The blunder resulted since tantalum and niobium have similarities that are always found together and are highly challenging to separate.
- Heinrich Rose, a German chemist, identified and named niobium in 1844 when he synthesized two new acids, niobic acid, and pelopic acid, from columbite and tantalite materials. These acids are extremely similar to one another.
- Christian Wilhelm Blomstrand, a Swedish scientist, was the very first to isolate metallic Nb in 1864.
- In 1866, a Swiss chemist named Jean Charles Galissard figured out that these were two unique compounds made from two different elements.
Occurrence of Niobium
- Nb does not occur freely in nature. It is the 34th most prevalent element in the Earth’s crust and makes up 0.002 percent of the crust.
- Nb is mostly found in minerals which include columbite (Fe, Mn)Nb2O6 and columbite-tantalite (or coltan, (Fe, Mn)(Ta, Nb)2O6) among others.
- Brazil and Canada are the biggest miners of Nb.
Isotopes Of Niobium
Nb has only one naturally occurring stable isotope.
| Isotope | Natural abundance (atom %) |
|---|---|
| 93Nb | 100 |
Elemental Properties of Niobium
| Electronic Configuration | [Kr] 4d45s1 |
| Atomic Number | 41 |
| Atomic Weight | 92.906 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 5, 5, d-block |
| Density | 8.57 g.cm -3 at 20 °C |
| Ionic radius | 0.070 nm (+5) ; 0.069 nm (+4) |
| Van der Waals radius | 0.143 nm |
| Electron shells | 2,8,18,12,1 |
| Electrons | 41 |
| Protons | 41 |
| Neutrons in most abundant isotope | 52 |
Physical Properties of Niobium
- Nb is a grayish metallic, incredibly soft metal with atomic number 41.
- It has a melting point of 2477°C (4491°F) and a boiling point of 4741°C (8566°F).
- The density of Nb is 8.4 grams per cubic centimeter.
- Nb has a [Kr]4d45s1 electrical structure, with electrons in the outermost shells. This is unusual for elements in Group 5.
- It is recognized as a ferromagnetic element because of its unusual crystalline structure and internal electronic configuration; it becomes magnetic once subjected to an external magnetic field.
- Nb is malleable, allowing it to be easily hit into sheets without cleavage, and ductility, which makes it possible to draw thin wires from it.
- Nb serves as an excellent electrical conductor. Because electrons in iron are free to move around they are able to carry electrical charge from one end to other.
- Nb is an effective thermal conductor as well. Heat causes a metal’s particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Nb has high corrosion resistance.
| Color/physical appearance | Silvery white |
| Melting point/freezing point | 2477°C, 4491°F, 2750 K |
| Boiling point | 4741°C, 8566°F, 5014 K |
| Density | 8.57 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
Chemical Properties of Niobium
When Nb is exposed to air, it normally forms layers of dielectric oxides, the color of which changes with thickness. Common colors include blue, green, and yellow.
Chemical Reaction of Niobium
- The reaction of Niobium with Air
Nb doesn’t react with air under normal circumstances.
- The Reaction of Niobium with water
Nb doesn’t react with water under normal circumstances.
- The Reaction of Niobium with the halogens
Nb reacts with the halogens to form Nb (V) halides when it gets heated.
Nb reacts with fluorine, F2, to form the halide niobium (V) fluoride, NbF5, when it is heated.
2Nb (s) + 5F2 (g) → NbF5 (s) [white]
Nb reacts with chlorine, Cl2, to form the halide niobium (V) chloride, NbCl5, when it is heated.
2Nb (s) + 5Cl2 (g) → NbCl5 (l) [yellow]
Nb reacts with bromine, Br2, to form the halide niobium (V) bromide, NbBr5, when it is heated.
2Nb (s) + 5Br2 (g) → NbBr5 (s) [orange]
Nb reacts with iodine, I2, to form the halide, niobium (V) iodide, NbI5, when it is heated.
2Nb (s) + 5I2 (g) → NbI5 (s) [brass coloured]
- The Reaction of Niobium with acids
At room temperature, Nb is unlikely to have any effects from multiple acids, but it does dissolve in hydrofluoric acid HF, or a mixture of HF and nitric acid, HNO3.
Uses of Niobium
- Because of its superconductive qualities, exceptionally high melting point, corrosion resistance, and wear resistance, Nb is widely used in industry and daily life. Some of these are discussed here:
- Used In Aerospace Industry: High-purity Nb is utilized in the aerospace and space sectors. Nb and tantalum alloys offer good thermal strength, thermal resistance, and processing qualities. These are often used in the production of airplane parts and gas turbine blades. Nb alloys are employed in the production of jet engines.
- Used In Metal Industry: Nb is frequently utilized in the industry due to its corrosion resistance and high melting point (2477°C). Adding a small percentage of Nb to steel can boost its yield strength by more than 30%. As a result, adding Nb to steel may boost not only its tensile strength but also its toughness, high-temperature oxidation resistance, and corrosion resistance. This makes it an ideal material for use in steel production since it prevents corrosion and increases the strength of steel products. It is also frequently added to alloys such as stainless steel to boost hardness and endurance. Furthermore, Nb is utilized as a fluxing agent for welder rods, which helps to lower the surface tension between two metals that are being welded together.
- Used In Nuclear Reactors: Nb alloys are also exceptionally radiation-resistant. This provides Nb, along with other refractory elements such as tungsten and molybdenum, with important applications in the nuclear sector. Because of their extraordinarily low neutron absorption cross-sections and great resistance to liquid metals, niobium-zirconium alloys are frequently employed as structural elements in nuclear reactors. Nb is also utilized in high-temperature reactor irradiation-resistant equipment.
- Used In Superconductor Material: Some Nb compounds and alloys possess a substantial superconducting transition temperature that is commonly utilized in the production of commercial superconductor technology such as superconducting generators, high-power accelerator magnets, superconducting magnetic energy storage, and magnetic resonance imaging equipment, among others. Superconductor materials such as niobium-titanium and niobium-tin are frequently employed in medical diagnostic magnetic resonance imagers and nuclear magnetic resonance devices for spectral lines.
- Used In Medical Field: Nb is used in the medical field for its excellent resistance to corrosion, oxidation, biocompatibility, and nontoxicity. It is widely used to produce hearing aid prosthetic devices, orthopedic implants, pacemakers, and other cardiovascular devices.
Nb is also used to manufacture radiation shielding materials, such as niobium foil, which can be utilized to shield employees against ionizing radiation that is produced in medical facilities and nuclear reactors. It is also used in hospital X-ray imaging machines to protect patients by blocking potentially harmful rays.
Health Effects Of Niobium
- Nb and its derivatives are potentially poisonous (Nb dust causes eye and skin irritation), however, there have been no reports of their poisoning humans. Aside from assessing its concentration, no human research on Nb has been conducted.
- Large levels of Nb can cause gastrointestinal problems such as nausea and vomiting.
- Nb is mostly preserved in the lungs and secondary in the bones after inhalation. It interferes with calcium’s role as an enzyme activator.
Environmental Effects of Niobium
- Mining and extraction of Niobium can also have a severe impact on the environment due to the discharge of toxic chemicals and pollutants into the air and water.
- Furthermore, disposing of Nb waste can be difficult because the metal can take millennia to decay organically.
References
- https://www.rsc.org/periodic-table/element/41/niobium
- https://www.lenntech.com/periodic/elements/nb.htm
- https://www.chemicool.com/elements/niobium.html
- https://byjus.com/chemistry/niobium/
- Lide, David R. (2004). “The Elements”. CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. pp. 4–21. ISBN 978-0-8493-0485-9.
- Nowak, Izabela; Ziolek, Maria (1999). “Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis”. Chemical Reviews. 99 (12): 3603–3624. doi:10.1021/cr9800208. PMID 11849031
- https://www.sciencedirect.com/topics/materials-science/niobium
- https://www.nature.com/articles/nchem.2164
