Nuclear magnetic resonance (NMR) spectroscopy is a physicochemical technique for determining the structural properties of molecules. It is described as the study of molecules by recording radiofrequency electromagnetic radiation interactions with the nuclei of molecules placed within a strong magnetic field. Zeeman, a Dutch physicist, first noticed the peculiar behavior of some nuclei when exposed to a strong magnetic field near the end of the nineteenth century. This phenomenon is now known as the “Zeeman effect.” The “Zeeman effect” was only applied in practice in the 1950s, when NMR spectrometers were commercially available.
NMR spectroscopy is based on the physical phenomena of magnetic resonance, which was discovered in 1938 by Isidor I. Rabi. Two research groups separately obtained the first successful measurements of NMR in condensed matter in the 1940s. The two groups’ primary investigators, Felix Bloch of Stanford University and Edward M. Purcell of Harvard University, were jointly awarded the Nobel Prize in Physics in 1952 for their contributions to magnetic resonance.
Interesting Science Videos
What is NMR spectroscopy?
Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical chemistry technique used in quality control and research to determine the quantity, purity, and molecular structure of a material. NMR Spectroscopy, which is based on the nuclear magnetic resonance phenomenon, offers comprehensive information on the structure, reaction state, and chemical surroundings of molecules.
In addition to studying molecular dynamics and interactions, NMR spectroscopy has emerged as one of the most potent methods for determining the structural properties of chemical species.
Principle of NMR spectroscopy
NMR spectroscopy is a physicochemical investigation technique based on the interaction of radiofrequency radiation applied externally with atomic nuclei. During this contact, there is a net exchange of energy, which causes a shift in nuclear spin, an intrinsic feature of atomic nuclei.
The nuclear spin is characterized by a quantic value (I) that varies depending on the isotope under consideration. NMR spectroscopy can identify only atomic nuclei with I ≠ 0 ( such as 1H, 2H, 13C, and 15N). These NMR-active nuclei act as small magnets (magnetic dipoles) that can align with external magnetic fields (a process known as magnetization). The magnetogyric ratio (γ), whose value depends on the isotope, defines the force of the small magnets.
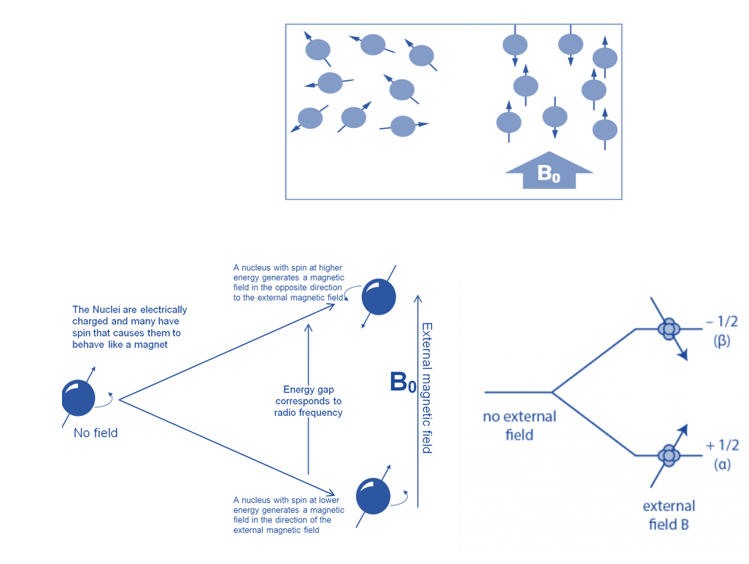
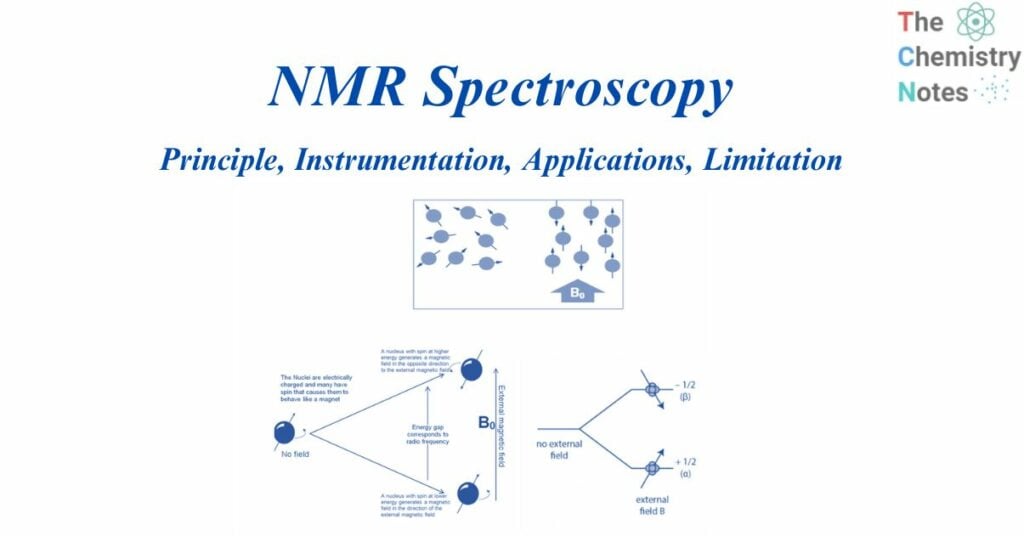
When aligned to an external magnetic field (B0), nuclear spins of some NMR-active nuclei can assume two distinct orientations. One orientation corresponds to the lowest energy level of the nucleus (parallel to the external magnetic field), while the other corresponds to the greatest energy level of the nucleus (antiparallel to the external magnetic field). When radiofrequency is applied to nuclei, magnetic resonance occurs. This produces energy level transitions, which result in changes in nuclear spin orientation. The difference in energy levels (E) is affected by the magnetic field and the magnetogyric ratio and influences the technique’s sensitivity.
When the spin returns from a high energy state to the ground state, it emits radiofrequency energy, producing a characteristic signal known as free induction decay (FID), which is detected by the detector. This FID is then converted into an NMR spectrum, which is a plot of intensities vs frequencies.
How does NMR spectroscopy work?
The NMR instrument is made up of three distinct elements. First, a superconducting magnet produces an external magnetic field surrounding the sample. A spectrometer, on the other hand, sends and receives radio waves. Finally, a computer operates the instrument and processes data.
NMR, in general, obtains a spectrum from the magnetic characteristics of atomic nuclei in order to analyze and distinguish atomic nuclei. The magnetic field of the instrument aligns the active nuclei in a sample prepared by dissolving 2 to 50 mg of the substance in a liquid. Radio frequencies correspond to the energy transition from the ground to an excited state, and when a nucleus returns to the ground (lower-energy) state, it emits radiation of the same frequency.
The intramolecular magnetic field, which is unique to each molecule, can provide information on the sample’s structure and functional groups.
This approach describes a molecule’s reaction circumstances, structure, chemical environment, and dynamics.
Instrumentation of NMR spectroscopy
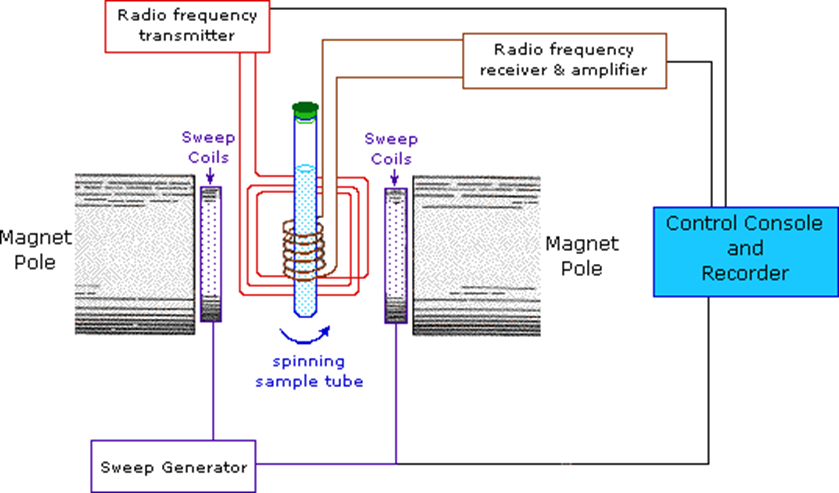
Fig: Instrumentation of NMR Spectroscopy
Image source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/nmr/nmr1.htm
Sample holder
In NMR spectroscopy, a typical NMR sample cell is 8.5 cm long and 0.3 cm in diameter. The sample holder should be chemically inert, long-lasting, and Rf radiation-transparent.
Glass tubes are often strong, practical, and inexpensive. For high-resolution spectra, the sample must be in a liquid or solution state.
Magnet
The magnet should produce a uniform magnetic field. The magnetic field strength is large because chemical changes are related to field strength. Two criteria are critical in magnet design:
i. field homogeneity or uniformity and field strength consistency
ii. maximum achievable field strength.
Permanent and conventional electromagnets are commonly utilized in spectrometers operating at frequencies ranging from 100 to 230 MHz.
Magnetic coil
A pair of coils (Helmholtz coils) positioned parallel to the magnet face can be used to change the applied field over a short range. The field intensity varies automatically and linearly over time, and this variation is synchronized with a linear device or a chart recorder.
Sweep generator
It alters the strength of a previously applied magnetic field. The precession frequency of a nucleus must be equal to that of the applied field or radiofrequency radiation in order for the nucleus to resonate. This can be accomplished by:
a. Frequency sweep method: The frequency sweep approach is used to resonate the nucleus. The frequency of the radiofrequency radiation is altered so that it equals the resonance frequency or the precession frequency.
b. Field Sweep Method: In this method, the frequency of the radiofrequency radiation is held constant while the precession frequency is varied by varying the applied magnetic field. It is preferable to radiofrequency radiation because it is easy to alter the HO.
Radiofrequency generator
A radio frequency generator is used to generate radio frequency. Normally, a fixed oscillator with a capacity of exactly 60 MHz is employed.
To maximize radiofrequency radiation interaction with the sample, the oscillator coil is looped around the sample container. The coil is wound perpendicular to the applied magnetic field.
Detector and recorder
The coil was used to direct the radio frequency signal generated by the resonating nuclei. The electrical signal generated in the coil must be amplified before it can be recorded. The detector assists in determining the unabsorbed radio frequencies. The NMR signals received by the RF detector are recorded by the recorder.
Types of NMR Spectroscopy
1H NMR (proton NMR)
The 1H (also known as proton) NMR spectroscopy is limited to ordinary hydrogens or protons. It is the most often used in laboratories. Because most organic compounds contain numerous hydrogen atoms, and the hydrogen atoms absorb energy of different wavelengths depending on their bonding environment, proton NMR spectra provide a wealth of information about molecular structure.
It gives the information:
- the number of distinct hydrogens in the compound
- Unique hydrogen ratios
- Each hydrogen’s chemical surroundings
- Hydrogens in close proximity
Carbon-13 magnetic resonance spectroscopy
C-13 NMR spectroscopy is used to analyze the carbons in a molecule. Although it is less prevalent than H-1, it is nevertheless utilized in organic chemistry laboratories. Natural carbon is almost entirely constituted of the carbon-12 isotope, which has no magnetic moment and hence cannot be detected by NMR techniques. Carbon-13 (13C) atoms, which account for around 1% of all carbon atoms, do, however, absorb radio-frequency waves in a way comparable to hydrogen. As a result, 13C NMR is feasible, and the technique yields useful information on the structure of the carbon skeleton in organic compounds. Because just one out of every hundred carbon atoms in a molecule contains a 13C isotope, and 13C atoms absorb electromagnetic radiation very poorly, 13C NMR signals are approximately 6,000 times weaker than proton signals. C-13 NMR can provide the following data:
- The total number of distinct carbons in the compound
- The compound’s chemical environment
NMR Active nuclides
Aside from 13C and 1H, there are numerous additional nuclei (or nuclides) that are NMR active. A nuclide must have a nonzero angular momentum quantum number (I) in order to be NMR active. It must also have a high enough natural abundance to be present in a sample containing the element in order to be useful. When abundance is low, the technique is less sensitive (for example, 13C NMR is substantially less sensitive than 1H NMR since the abundance of the isotope 13C is only around 1%, whereas 1H is approximately 100%).
Other typical nuclides for NMR include 15N, 19F, 31P, and 57Fe, all of which have the standard angular momentum quantum number I = 1/2.
Techniques for NMR Spectroscopy
1. Resonant Frequency
It corresponds to the absorption energy and signal intensity, which are related to the strength of the magnetic field. When put in a magnetic field, NMR active nuclei absorb electromagnetic radiation at a frequency characteristic of the isotope.
2. Spectral Acquisition
A nuclear magnetic resonance response is acquired after the excitation of the sample with a radiofrequency pulse. Since it is so weak, only radio receivers with high sensitivity can pick it up.
Chemical shift in NMR spectroscopy
The precise resonance frequency of the energy transition is determined by the nucleus’ effective magnetic field. This field is influenced by electron shielding, which is changed by the chemical environment. As a result, information about the chemical environment of the nucleus can be extracted from its resonance frequency. In general, the greater the resonance frequency, the more electronegative the nucleus. The frequency shift is affected by other factors such as ring currents (anisotropy) and bond strain.
So, the position of each peak is generally assessed in relation to the protons’ absorption in the chemical tetramethylsilane, (CH3)4Si. Tetramethylsilane is an inert liquid that is added in trace amounts to the substance under investigation. All 12 of its hydrogen atoms absorb at the same place, resulting in a single sharp peak with an arbitrary positional value of zero. This peak is then utilized as a starting point for all subsequent peaks in the spectrum. The hydrogen atoms in the molecule being analyzed often appear to the left of the reference peak because they absorb more energy than the tetramethylsilane hydrogens.
Chemical shift is defined as the difference between the resonance frequency of the spinning protons and the reference molecule’s signal. In absolute terms, the chemical shift is defined by the frequency of the resonance expressed in relation to a standard substance, which is defined to be at 0 ppm. The scale is simplified by expressing it in parts per million (ppm) and is independent of the spectrometer frequency.
Chemical shift = frequency of signal- frequency of reference/ Spectrometer frequency X 106
The chemical shift of hydrogen atoms is the most essential piece of information offered by NMR spectroscopy, as it informs a lot about the nature of the bonds that surround the hydrogen. For example in bromoethane, an electronegative bromine atom attracts electrons away from the carbon and hydrogen atoms. As CH2 hydrogens are closer to the bromine atom, they are more strongly influenced than CH3 hydrogens and so have a higher chemical shift. All three hydrogens in the CH3 group are subjected to the same local magnetic field, resulting in the same chemical shift. So these hydrogens are referred to as chemically equivalent. Similarly, the two hydrogens in the CH2 group are also equivalents.
Upfield and downfield NMR Spectra
The names upfield and downfield relate to the regions with lower and higher values on the chemical shift scale, respectively.
The same type of nucleus can provide signals with varying chemical shift values. These chemical shifts differ because the magnetic field experienced by a specific nucleus is heavily influenced by its nearby chemical environment. The movement of electrons around a nucleus generates tiny magnetic fields that counter the applied external field. This “shielding” effect is proportional to the nucleus’s electrical density. As a result, the effective magnetic field operating on the nucleus is reduced, affecting the Larmor frequency. When there is a high electronic density surrounding the nucleus under consideration, the shielding effect is strong, the Larmor frequency lowers, and the chemical shift (which goes upfield) reduces.
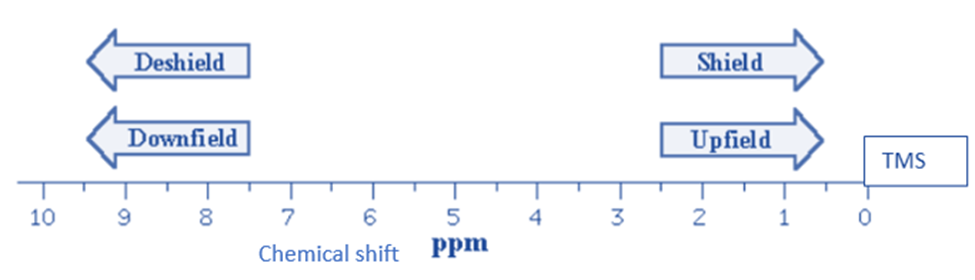
Methyl groups and aliphatic compounds have hydrogen nuclei that are well-shielded and have typical chemical shift values upfield. Hydrogen nuclei linked to electronegative atoms (such as oxygen or nitrogen) or near electronegative groups (such as carboxylic acids or aldehydes), on the other hand, are unshielded and exhibit chemical values situated downfield.
When the electronic density near the nucleus is low, the shielding effect is low, the Larmor frequency increases and the chemical shift decreases (it travels downfield).
Larmor frequency:
When atomic nuclei are subjected to a magnetic field, nuclear magnetic dipoles do not move statically with the magnetic field B0 but rather move like a spinning top around an axis parallel to the direction of the field (precession movement). The magnetogyric ratio and the magnetic field dictate the frequency of this precession movement, known as the Larmor frequency (vL).
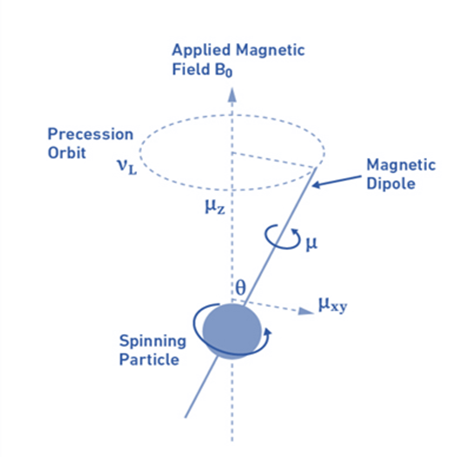
Factors affecting NMR peaks
- If a proton is linked to an electronegative atom or group, it is said to be deshielded. The larger the atom’s electronegativity, the greater the deshielding causes to the proton. The chemical shift value () increases as the deshielding of a proton increases.
- Some protons in overcrowded molecules may occupy sterically hindered positions, resulting in van der Waal’s repulsion. The electron cloud of a bulky group (hindering group) will tend to reject the electron cloud around the proton in such compounds. As a result, such a proton will be deshielded and resonate at a little greater chemical shift value than would be expected in the absence of this effect.
- Electron-donating substituents shield aromatic protons by increasing electron density on the benzene ring. Because the electron density is larger at ortho and para positions due to the +M effect, the order of shielding is ortho > para > meta. The electron-withdrawing substituents, on the other hand, result in the deshielding of all aromatic protons. Because the electron-withdrawing effect is greatest at ortho and para positions, the order of deshielding is ortho > para > meta.
- The most commonly used solvent for NMR spectroscopy is chloroform (CDCl3). Deuterium-labeled substances like deuterium oxide (D2O) are also employed as NMR solvents. The polarity and magnetic susceptibility of all of these solvents vary greatly. As a result, the NMR spectrum recorded in one solvent may deviate slightly from that recorded in another. Changing the solvent normally causes a minor shift in the NMR signals of protons linked to carbon, unless there is strong bonding or dipole-dipole interaction.
Spin spin coupling in NMR spectroscopy
Spin-spin coupling is the interaction of the spin magnetic moments of different sets of H atoms in the molecule under study. A minimum of two sets of protons must be present in adjacent places.
The magnetic spins of these resonating nuclei interact with one another, influencing their precession frequencies.
Thus, the chemical shift values depend on the effective magnetic field (Beff) experienced by nearby protons due to magnetic spins. The nature of the peaks in the NMR spectra is also changed, in addition to the chemical changes.
Peak splitting in NMR spectroscopy
The number of peaks (the number of lines) in NMR signals might vary. This is referred to as signal splitting or multiplicity.
Signal splitting is undoubtedly the most distinctive and fundamental property that distinguishes NMR spectroscopy as a powerful tool for structure determination.
Hydrogens on the same carbon (geminal hydrogens) or on neighboring carbons (vicinal hydrogens) cause the splitting. Only non-equivalent protons are capable of splitting the signal of the provided proton(s).
Peak splitting is the process by which the spins of resonating protons cause the peaks on the NMR spectrum to multiply. The NMR signal splitting provides accurate information on the number of neighboring protons in a molecule. The multiplicity of the peaks in the NMR spectrum can be calculated using a formula.
2nI + 1
n = Number of protons in close proximity i.e., neighboring protons.
I = proton spin number
We can rewrite the formula as n+1 since the value of I is always 1/2.
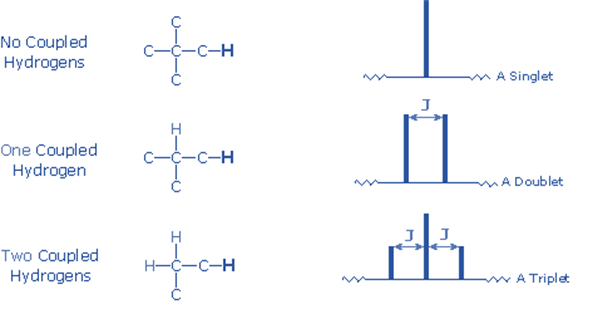
If there is only one neighboring proton, the NMR signal is split into a doublet; if there are two adjacent protons, the signal is split into a triplet.
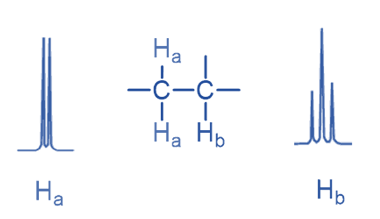
Here signal of Ha is split into a doublet due to the presence of a single Hb while the Hb signal is split into a triplet due to the presence of two Ha.
Only non-equivalent protons can split the signal. As enantiotopic and homotopic protons are chemically equal, signal splitting is not seen for either of them. For example, methane and ethane, both have equivalent protons that do not split, and the signal appears as a singlet. Similarly, 1,2-dichloroethane is a molecule in which the hydrogens are equivalent, resulting in only one singlet.
The general guidelines that follow crucial criteria and properties for spin 1/2 nuclei:
1) Nuclei with the same chemical shift (referred to as isochronous) do not show spin-splitting. They could be spin-coupled, but the splitting cannot be immediately noticed.
2) Nuclei separated by three or fewer bonds (e.g., vicinal and geminal nuclei) are frequently spin-coupled and exhibit mutual spin-splitting of the resonance signals (same J’s), provided their chemical shifts are different. Longer-range coupling may be observed in molecules with rigid atom structures.
3) The magnitude of the observed spin-splitting is determined by a number of parameters, including the coupling constant J (in hertz). J is the same for both partners in a spin-splitting interaction and is unaffected by the strength of the external magnetic field.
4) The n+1 rule can estimate the splitting pattern of a particular nucleus (or group of equivalent nuclei), where n is the number of neighboring spin-coupled nuclei with the same (or extremely similar) Js. If there are two spin-coupled nuclei next to each other, the measured signal is a triplet (2+1=3); if there are three spin-coupled neighbors, the signal is a quartet (3+1=4). The center line(s) of the splitting pattern are always stronger than those on the periphery.
Applications Of NMR Spectroscopy
- Organic, organometallic, and biological compounds are identified and structurally elucidated by using NMR spectroscopy.
- In biophysics and molecular biology, NMR spectroscopy is used to investigate the structure, dynamics, and molecular interactions of biomolecules such as peptides, proteins, nucleic acids, carbohydrates, and others.
- NMR spectroscopy is also employed in biological fluid analysis to acquire metabolic profiles related to disorders (metabolomics), as well as in NMR imaging techniques for medical diagnosis.
- NMR fingerprint analysis is used to verify the quality or authenticity of food, wine, and cannabis samples.
- In the NMR spectrum, each functional group has a distinct signal. The functional group in the compound can be identified by observing the chemical shift of the substance.
- It is feasible to place hydrogen at a suitable position in the formula and so determine the structure of a given molecule by observing the chemical shift values and splitting of the signal of protons under different conditions.
- NMR spectroscopy may be used to assess molecule conformation in solutions as well as analyze physical features at the molecular level such as conformational exchange, phase shifts, solubility, and diffusion once the basic structure is understood.
- NMR spectroscopy can quantify mixtures containing known chemicals. For unknown substances, NMR can be utilized to compare against spectral libraries or to derive the basic structure directly.
- NMR spectroscopy is used in pharmaceutics to examine the structure, dynamics, and molecular interactions of pharmaceuticals for drug discovery, quality control, and purity determination.
- It is used in petrochemistry for rock material analysis to determine the appropriateness of an oil reservoir for extraction, solid-state NMR composition analysis of petroleum derivatives, and product quality control.
- NMR spectroscopy is also used for the characterization of novel materials.
- NMR spectroscopy is used to investigate dynamic features of molecules such as conformational isomerism, molecular asymmetry, hydrogen bonding, and so on.
- It is used to determine the optical purity.
- NMR spectroscopy is used to investigate drug-receptor interactions.
Limitations of NMR spectroscopy
- NMR spectroscopy is a crucial method in organic chemistry. However, there are some limitations of this method that should be considered while employing it.
- NMR spectroscopy is a versatile approach, however, it sometimes fails due to its low sensitivity. This is a significant drawback when investigating metabolomics or other complex reactions.
- Drift in the magnetic field has a significant impact on NMR spectroscopy, resulting in deformed lines and spectral leakage. This can make interpreting results exceedingly challenging.
- The sample must be NMR active.
Reference
- https://www.britannica.com/science/chemical-compound/Proton-magnetic-resonance-spectroscopy.
- https://microbenotes.com/nuclear-magnetic-resonance-nmr-spectroscopy/.
- https://byjus.com/chemistry/nmr-spectroscopy/.
- https://www.slideshare.net/RawatDAGreatt/nmr-40157983.
- https://chem.ch.huji.ac.il/nmr/whatisnmr/whatisnmr.html.
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/nmr/nmr1.htm.
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_(Barron)/04%3A_Chemical_Speciation/4.07%3A_NMR_Spectroscopy
