Nylons are a class of polymers known as polyamides that are created by heating, high-pressure reactions of carbon-based compounds present in coal and petroleum. Nylons are among the most prevalent polymers used as textiles. It is highly regarded for its unique mix of low friction qualities and robust mechanical, chemical, and thermal properties. It is a silk-like thermoplastic, typically derived from petroleum, that may be melt-processed into fibers, films, or forms.
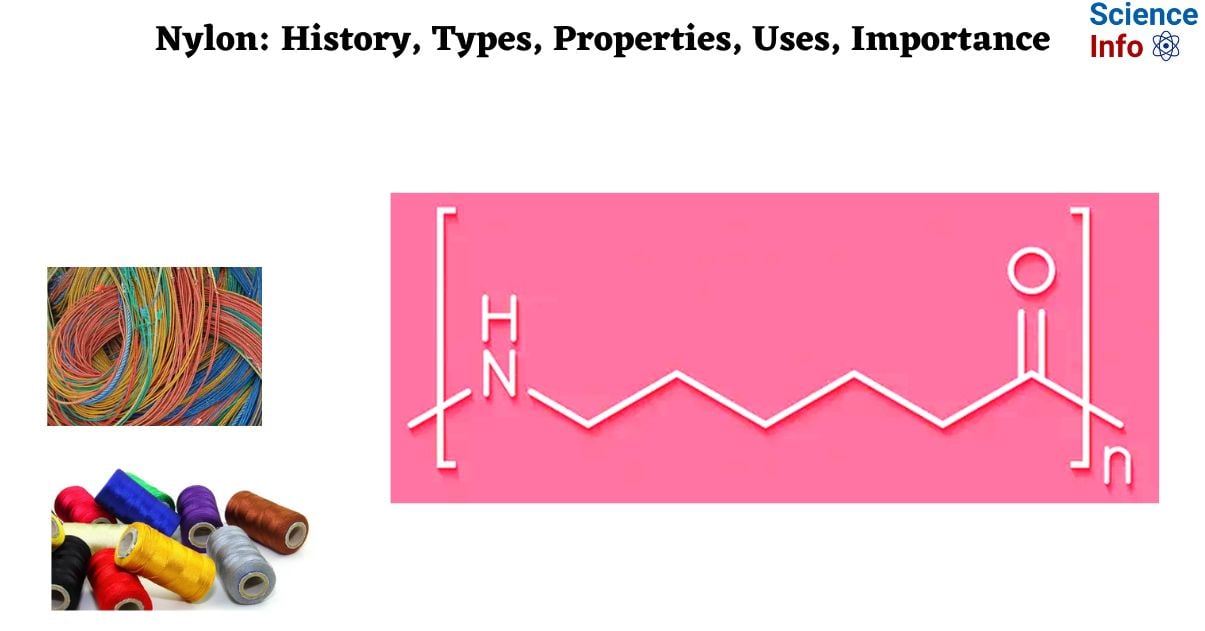
Nylon polymers can be blended with a variety of additives to achieve a wide range of properties. It was initially developed in a DuPont Chemicals laboratory on Feb. 28, 1935, however, it wasn’t made accessible to the general public until 1940.
Interesting Science Videos
History and Discovery of Nylon
- A research team led by American scientist Gerard J. Berchet of Wallace Carothers’ research division at DuPont (E.I. du Pont de Nemours and Company) in Delaware created nylons on February 28, 1935.
- Nylon, a prototype synthetic fiber made entirely from coal-derived building blocks in the presence of both water and air, was also the first polymer to attain commercial success.
- Originally, in 1938, it was used to produce nylon-bristled toothbrushes, which were later transformed into fabric appropriate for women’s stockings in 1940.
- It was created to serve as an artificial alternative for silk but was used in parachutes when the United States entered World War II in 1941, which made stockings difficult for people to get by until the war ended.
- There is no clear reason behind the etymology of nylon, however, DuPont revealed that the name was initially supposed to be “No-Run” where “run” implies “unravel”, but there was no genuine foundation for the claim at the time, so it had to be changed to improve the word’s clarity.
- One prevalent theory is that the term “nylon” was derived from “New York and London,” the hometowns of the chemists responsible for the material’s production. Unfortunately, there is no evidence to suggest nylon was named after New York or London.
Synthesis of Nylon
- The nylons are constructed up of long-chain molecules, or Polymers, which are linked together with smaller building components, or monomers.
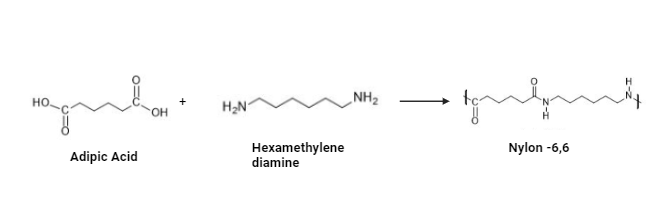
- The nylons are made up of long-chain molecules, or polymers, which are linked together with smaller building components, or monomers. The majority of nylons are synthesized by reacting two kinds of building blocks: a diamine (a chemical base) and a dicarboxylic acid.
- A unique class of linkages known as amide bonds—also called peptide bonds—connects these monomers to form extraordinarily long chains. This leads to the polymer’s classification as a polyamide (PA).
- It is difficult to achieve a precise one-to-one ratio of diamine (base) and diacid while manufacturing nylon and the chemical reaction may stop before the chains of polymers are long enough. To address this issue, a crystalline, solid “nylon salt” can be created at room temperature by utilizing a precise one-to-one ratio of acid and base to neutralize each other.
Types of Nylon
Nylons are designated by a number, which indicates the number of carbon atoms between the amine and acid groups, as well as how the polymer was formed. Nylons can also be reinforced with glass or mixed with other technical plastics to improve performance and material properties. There are a lot of Nylons but we will take a look at five types of Nylons:
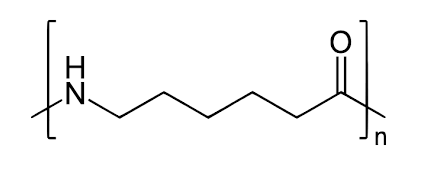
Nylon 6
- Nylon 6 filaments are exceptionally flexible fibers that are widely utilized in textiles and durable industrial applications such as clothing, ropes, and industrial wires.
- These synthetic fibers have a seamless, glass-like texture that provides excellent abrasion resistance. Nylon 6 often produces higher-quality final part dimensions.
- Nylon 6 fibers are durable, with excellent tensile strength, flexibility, and sheen.
- As a synthetic fiber, nylon 6 is typically white; however, it can be dyed in a solution bath before manufacture for a variety of colors.
- Nylon 6 is used as a building material in a variety of industries, including automotive, electronic and electrotechnical, aerospace, apparel, and medicine.
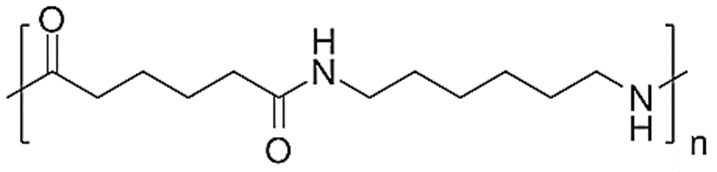
Nylon 6,6
- Since Nylon 6,6 has more crystals than Nylon 6, it has higher tensile, flexural, and stiffness moduli.
- Because of this, it’s the perfect material for things like carpets, airbags, and luggage that need to be strong, stiff, and chemically resistant.
- Because of its greater melting point, nylon 6,6 is more susceptible to mold shrinkage.
- Nylon 6,6 is used in the manufacture of wear pads, sliding bearings, and guide wheels.
Nylon 12 (PA 12)
- PA 12 or Nylon 12 is a versatile polymer that has multiple additional applications. It is recognized for its resilience, tensile strength, impact strength, and ability to stretch without breaking.
- It has been used as an injection molder for years due to its above-mentioned mechanical properties.
- It has lately been accepted as a standard material in additive manufacturing procedures for the creation of functional components and prototypes.
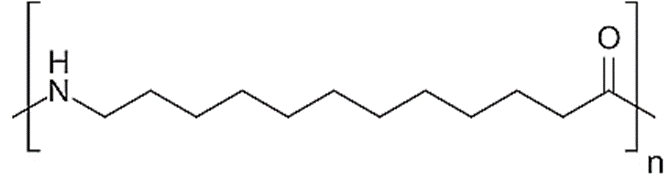
Nylon 1,6 (Polyamide 1,6)
- Acid catalysis can be used to generate nylons from dinitriles. This method, for example, can be used to generate nylon 1,6 from formaldehyde, adiponitrile, and water.
- Because of the high density of amide residue in the polymer, it has a significant moisture absorption.
- Fabrics made with nylon 1/6 are not commonly utilized.
Nylon 4,6
- Nylon 4,6 was engineered to be used at a higher temperature compared to other types of nylon.
- The advantages of nylon 4,6 include a higher thermal distortion temperature than nylon 6 and nylon 6,6.
- It also has better crystallinity, which improves chemical resistance, particularly to acidic salts, and reduces cycle times.
- Therefore a result of this, its application areas comprise engine components including transmissions, brakes, and air cooling systems.
Nylon 510
- Nylon 510, produced using Penta-methylene diamine (PDA) and Sebacic acid (C10H18O4), was cited in the Carothers patent for nylon 66.
- It has higher standards of strength and durability but is expensive to manufacture.
- As it has a higher cost of production its application as fabric is not viable.
- It is only used for industrial and scientific purposes.
General Properties
| Specific Gravity | 1.14 gm/cm3 |
| Tensile Strength | 76 MPa |
| Tensile Modulus | 2758 MPa |
| Stiffness | 20-24 gm/density |
| Tenacity | 4-9 gm/den dry; 90% wet |
| Impact Strength | 5.0 kJ/m² |
| Typical Mold Shrinkage | 1.3 % |
| Resiliency | Excellent |
| Melting Point | 256 °C (492 °F) |
| Abrasion Resistance | Excellent |
| Dimensional Stability | Good |
| Elasticity | The breaking extension is 20-40% |
Physical Properties of Nylon
- Despite its tiny fibers, nylon is robust and resilient enough to endure years of use. Its synthetic nature contributes to its resilience.
- Nylon is helpful for items that need plasticity as it can be molded in any form you want.
- Nylon is lustrous, which implies that it’s shiny. It can be exceedingly glossy, semi-lustrous, or dull, depending on its intended usage. This is why it is frequently employed as a fabric.
- Nylon has inherent high tensile strength and durability, making it excellent for applications requiring the material to withstand impacts.
- Below its melting point, nylon possesses amorphous areas that alternate with lamellar crystals. The amorphous portions provide flexibility to the nylon.
- Nylon is stretchy because, when heated above its melting point, it becomes an amorphous solid or viscous fluid in which the chains resemble random coils.
- Nylons are hygroscopic, which means they absorb or desorb moisture based on the surrounding humidity.
- Changes in moisture levels have a variety of consequences on the polymer.
- When dried, polyamide is a great electrical insulator.
- High-insulating characteristics cause static electricity on the fiber.
- Nylon dries quicker compared to other fabrics due to its hydrophobic properties.
Chemical Properties of Nylon
- Because of its strong bonds, which are composed of two incredibly strong molecules, nylon is resistant to most chemicals.
- Mineral acids damage nylon, causing it to degrade or virtually dissolve.
- It is ineffective in diluting acetate and formic acids. It is dissolved in highly concentrated formic acid.
- Nylon is susceptible to mineral-based acids however immune to dilute boiling organic acids.
- Nylon is highly inert to alkalis.
- Over time nylon loses its strength.
- Most of the organic solvents have limited or no effect on nylon.
- Nylon is unaffected by oxidizing and reducing bleaches, although chlorinated and oxidative solid bleaching agents can be harmful.
- Phenol meta-cresol and formic acid disintegrate the fiber, however, solvents used for removing stains and dry cleaning do not harm it.
Applications of Nylons
Nylon is utilized as a coating on several medical appliances. This material’s capacity to endure several chemicals and sterilization makes it an excellent choice for medical equipment. As a result, it is suitable for use in catheters (type of tubes), bandages, walkers, hospital beds, and syringes.
Nylon is also used in plastic machinery plants. It is durable, lightweight, and resistant to heat and chemicals, can even be utilized in machine parts. These components comprise screws, nuts, and bolts. Nylon is commonly used in the electronic sector for goods like circuit boards and power lines.
Nylon is also used as a replacement for various metals. Contrary to metal, nylon is affordable and pliable. As a result, purchasing and producing nylon-based products becomes less expensive. Furthermore, because nylon is more flexible than metal, cleaning instruments made of nylon may reach areas that metal tools cannot effectively clean.
Nylon is also applicable in some military equipment. Military gear including tents, bags, uniforms, and ropes can benefit from nylon’s strength and resilience to heat and chemicals. Nylon chips are melted, and then spun in a spinneret to create nylon fibers. Numerous microscopic holes in the spinneret allow the nylon fiber to alter in size and shape. The fibers can be weaved together to make ropes strong enough for industrial usage once they have cooled.
Environmental Effects of Nylon
The majority of nylon’s effects on the environment are detrimental. It requires a lot of energy to create nylon.
- Since it’s a synthetic polyamide. Nitrous oxide, a greenhouse gas that considerably contributes to global warming, is released during the manufacture of nylon. Furthermore, nylon does not biodegrade.
- Nylon may be recycled indefinitely, though. Designers may utilize nylon’s utility and improve the environment at the same time by using recycled textiles.
- Recycling still consumes a lot of energy, produces greenhouse emissions, and calls for the use of more dangerous chemical dyes.
Toxicity of Nylon
- As nylon polymers are non-reactive and generally regarded as non-harmful, the fabric is safe to use.
- Nonetheless, nylon can trigger allergic symptoms in some individuals who happen to be sensitive to petroleum-based compounds.
Video Reference
References
- https://www.bpf.co.uk/plastipedia/polymers/Polyamides.aspx
- https://www.newworldencyclopedia.org/entry/Nylon#Bulk_properties
- https://www.sciencehistory.org/stories/magazine/nylon-a-revolution-in-textiles/
- https://pslc.ws/macrog/nylon.htm
- https://sewport.com/fabrics-directory/nylon-fabric
- https://goodonyou.eco/material-guide-nylon/
- https://www.xometry.com/resources/materials/properties-of-nylon/
- https://sybridge.com/know-your-materials-nylon/
- https://www.millerplastics.com/nylon-properties-and-uses/

