Oganesson is a synthetic transition metal with an atomic number of 118 and is represented by the symbol ‘Og’ in the periodic table. It is silvery in appearance and belongs to the p-block of period 7 of the periodic table. It is the final element in the periodic table. Only tiny quantities of Oganesson have been successfully synthesized, hence there isn’t much known about it based on experimental data, but some qualities can be predicted using periodic table trends.
Oganesson is an extremely radioactive element that does not occur naturally, and is produced inside a laboratory setting and decays within milliseconds after being synthesized. This element, was formerly known as Ununoctium or eka-radon. A team lead by Yuri Oganessian, consisting of scientists from the Joint Institute of Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL), discovered element-118 in 2002, but did not reveal it until 2005. Oganesson has been named after Yuri Oganessian, who contributed to transactinide element research.
Interesting Science Videos
History and Discovery of Oganesson
- In accordance with Mendeleev’s naming for unknown elements, oganesson is known as eka-radon or element-118.
- The Danish physicist Neil Bohr had anticipated the oganesson in 1922, and eighty years later, it was successfully synthesized.
- In the year 2002, oganesson was created with the cooperation of an American-Russian team at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, under the direction of Yuri Oganessia.
- A group of researchers directed by Yuri Oganessian, comprising scientists from the Joint Institute of Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL), discovered element-118 in 2002, but did not announce their discovery until 2005 because the decay energy of oganesson-294 was the same as that of polonium-212m.
- Using a cyclotron, the researchers bombarded californium-249 atoms with calcium-48 ions, yielding the isotope Oganesson-294, which has a half-life of 0.9 milliseconds and three neutrons. The target atoms of californium were exposed to 16×1018 calcium ions.
- Since 2005, fewer than six atoms of oganesson have been synthesized in experiments around the world.
- The first isotope was produced in 2011.
- Following additional investigations, the IUPAC verified Oganesson’s claim in 2015.
- In 2016, the International Union of Pure and Applied Chemistry officially changed its name to oganesson in honor of Russian physicist Yuri Oganessian.
Occurrence of Oganesson
- Oganesson can be synthesized artificially. It’s a synthetic element that is extremely unstable. Its half-life is only a few seconds.
- Og is a synthetic radioactive metal formed by nuclear bombardment and has only been manufactured in trace amounts. And it is only found in specialized laboratories due to its rapid decay.
- A few oganesson atom were produced by bombarding 249Cf with 48Ca ions in a heavy ion accelerator.
- The experiments carried out at the Flerov Laboratory of Nuclear Reactions in Dubna, Russia, by researchers from the Joint Institute for Nuclear Research in Russia and the Lawrence Livermore National Laboratory in the United States, revealed that element 118 (oganesson, Og) was obtained. However, only one atom was discovered in the spring of 2002, followed by two more in 2005.
- The 2002 experiment involved shooting a beam of 4820Ca at 24998Cf. The experiment lasted four months and used a beam of 2.5 x 1019 calcium ions to create the single event thought to be the synthesis of element 118 (ununoctium) as the isotope 294118Uuo. Three neutrons were discharged during this procedure.
24998Cf + 4820Ca → 294118Og + 31n- Oganesson has a single isotope whose half-life is known fairly roughly: 294Og.
Elemental Properties of Oganesson
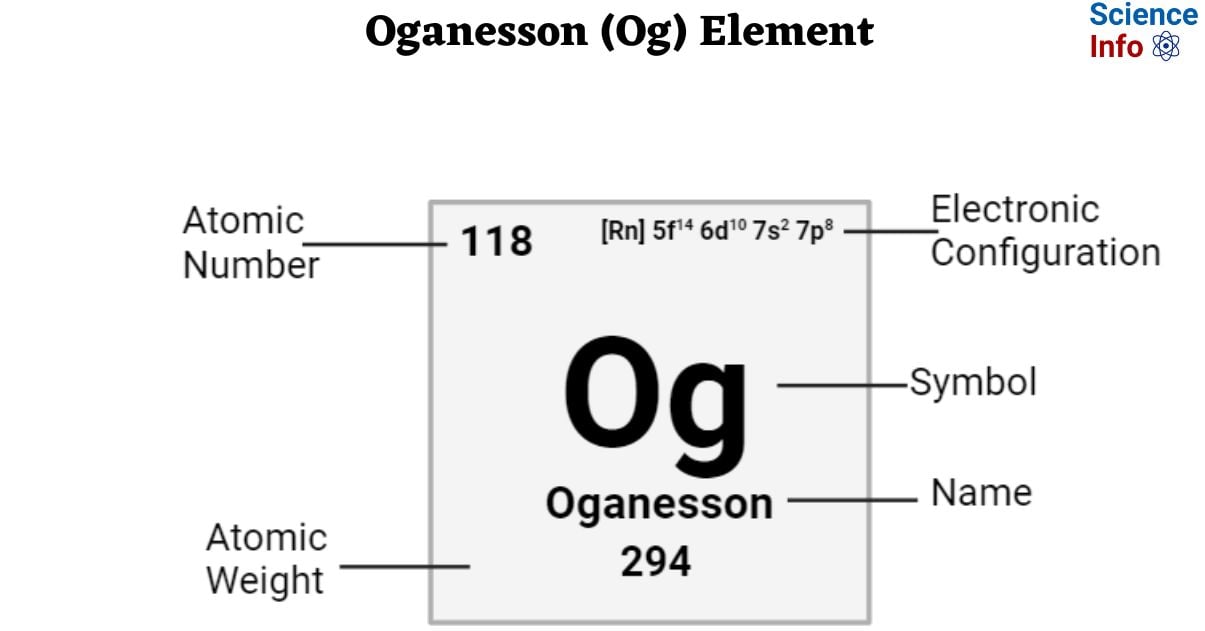
| Electronic Configuration | [Rn] 5f14 6d10 7s2 7p8 |
| Atomic Number | 118 |
| Atomic Weight | 294 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Transactinide, 7, p-block |
| Density | _ |
| Ionic radius | – |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 32, 18, 7 (estimated) |
| Electrons | 118 |
| Protons | 118 |
| Neutrons | 176 |
Isotopic Information of Oganesson
- Oganesson does not have any naturally occurring stable isotopes, but they can be created in a laboratory setting.
- All of the Og isotopes are unstable and radioactive.
- Oganesson-294 decays mostly through the emission of alpha particles, with less frequent spontaneous fission.
- Oganesson has one known isotope: 294Og.
- An additional isotope, oganesson-295, was discovered in an experiment conducted by the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany in 2011 but it has not confirmed by the scientific community yet.
- Og-294 has a half-life of about 0.89 milliseconds and is currently the most stable isotope of Oganesson known to scientists.
Physical Properties of Oganesson
- Oganesson’s instability makes it difficult to conduct an objective examination of its physical properties.
- Given its swift disintegration, only a few properties of Oganesson have been studied to date.
- It is a synthetic, super-heavy transactinide element.
- It is found in the 7th period, the 18th Group (noble gases), and the p-block of the periodic table.
- Oganesson was previously thought to be a gas under normal settings, however due to the relativistic effects of its atoms, it is now known that it exists in solid form under normal conditions.
- Considering its position on the periodic table, element 117 can be expected to be as noble gases.
- It has the highest atomic mass and number of any of the periodic table’s 118 elements.
- It is supposed to be oxidation-resistant.
- The isotope of Oganesson with the longest half-life is 0.89 milliseconds.
Chemical Properties of Oganesson
- Oganesson is a highly radioactive element. Its chemical properties have yet to be completely investigated. Isotopes have short half-lives, and the compounds they contain are highly volatile, making conclusive chemical analysis challenging.
- There have been no experimental measurements of Oganesson compounds, and all known predictions are theoretical. No oganesson compound has been created yet.
- It is the heaviest element in the 18th group of the periodic table.
- It is expected that oganesson, unlike the other members of Group 18, is a reactive metal.
- However, oganesson’s chemical characteristics are likely to be similar to other members of group 8, particularly radon.
- Oganesson compounds are expected to exhibit the most prevalent oxidation state of ‘0’.
Chemical Reaction of Oganesson
The reactions of oganesson are not conclusive.
- Reaction of Oganesson with Air
Because no atoms of oganesson have previously been created, its reactivity with air remains unknown. However, radon’s behavior (just above oganesson in the periodic table) suggests that it does not react with air.
- Reaction of Oganesson with Water
Since no atoms of oganesson were ever created, its interaction with water remains unknown. However, given the behavior of radon (just above oganesson in the periodic table), one would expect it to not react with water. It would most likely dissolve to some amount in water at 20°C (293 K).
- Reaction of Oganesson with Halogens
Considering no atoms of oganesson were previously created, its reactivity with the halogens is undefined. One would expect its behavior to be similar to that of radon (just above oganesson in the periodic)
- Reaction of Oganesson with Acids
Because no atoms of oganesson have ever been created, its reactivity with acids remains unknown. One would expect its behavior to be comparable to that of Radon (Rn) (directly above oganesson in the periodic table) and xenon (two places above).
Synthesis of Oganesson
- All elements with atomic numbers more than 100 can only be created through reactions in a particle accelerator, such as a cyclotron; they do not develop in a nuclear reactor.
- Californium-249 produces oganesson-294 when bombarded with calcium-48.
Uses of Oganesson
- Given that few atoms of this metal have been synthesized thus far, there are currently no specific or specialized applications for oganesson other than scientific research.
- A constant experimental study aimed at achieving an obvious conclusion requires a large number of atoms. Perhaps a few oganesson atoms have been produced thus far.
Health Effects of Oganesson
- Oganesson is a highly unstable chemical; when created, it swiftly decomposes into other elements, therefore it has no impact on human health. However, being a radioactive element it must be toxic.
Environmental Effects of Oganesson
- Oganesson’s environmental effects are negligible due to its short half-life (just a few seconds).
Video Reference
References
- https://periodic-table.com/oganesson/
- https://www.ccdc.cam.ac.uk/elements/oganesson/
- https://chemicalengineeringworld.com/oganesson-element-properties-and-information/
- https://www.thoughtco.com/ununoctium-facts-element-118-606613
- https://www.webelements.com/oganesson/chemistry.html
- https://www.chemicool.com/elements/oganesson.html

