Interesting Science Videos
Organic Compounds Definition
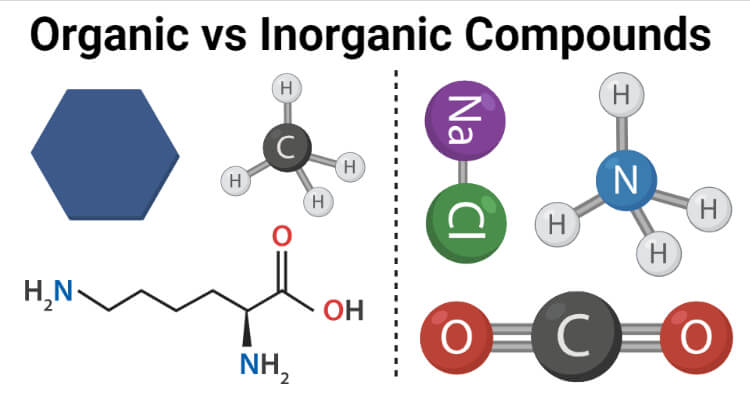
Organic compounds are chemical compounds composed of one or more carbon atoms bonded to other elements like hydrogen, oxygen, or nitrogen.
- Organic compounds consist of multiple carbon-hydrogen and carbon-carbon bonds that hold the structures together.
- Organic compounds are the result of the catenation property of carbon atoms as they can form chains of varying lengths with other carbon atoms.
- Most of the carbon compounds except carbonates, cyanides, and carbon dioxide are organic compounds.
- These are essential as all known life on Earth is based on organic compounds even though the organic compounds account for only a single percentage of the Earth’s crust.
- Organic compounds form a separate branch of chemistry involved in studies related to their properties, reactions, and synthesis known as organic chemistry.
- Organic compounds can be categorized into different groups depending on different properties. One of the major distinctions is natural and synthetic organic compounds depending on their source.
- Besides, these can also be divided into organometallic compounds and organophosphorus compounds depending on the presence of heteroatoms.
- Organic compounds are composed of covalent linkages, and other forms of linkages are mostly absent.
- Most organic compounds exist in the liquid state at room temperature, but these can exist in a solid and gaseous state as well.
- Even though organic compounds form the basis of life, living organisms can incorporate inorganic compounds in organic compounds via different processes in the carbon cycle.
- The primary source of organic compounds in living beings is photosynthesis, where green plants and algae utilize carbon dioxide and a hydrogen source to produce natural organic compounds.
- In the case of synthetic organic compounds, most of these are derived from petrochemicals composed of hydrocarbons. The petroleum hydrocarbons are, in turn, formed due to the degradation of organic matter under high pressure and temperature.
- Some of the common examples of organic compounds include glucose, lysine, methane, acetone, etc.
Inorganic Compounds Definition
Inorganic compounds are compounds consisting of two or more elements, usually other than carbons, that are linked together in definite proportions and lack carbon-hydrogen linkages.
- The concept of the inorganic compound was originated to differentiate organic compounds from other types of compounds.
- Even though most compounds composed of carbon are considered organic compounds, compounds like carbides, carbonates, cyanides, carbon dioxide, etc., are inorganic compounds.
- The studies related to inorganic compounds have been separated from organic compounds to form an isolated branch of chemistry called inorganic chemistry.
- Inorganic compounds can occur in living organisms in different forms and are often incorporated into organic compounds during the carbon cycle.
- Inorganic compounds lack carbon-carbon bonds, and long-chain carbon compounds do not occur in inorganic compounds.
- Inorganic compounds consist of elements that are linked together by ionic, metallic as well as covalent bonds. As a result of these bonds, inorganic compounds have different physical and chemical properties than organic compounds.
- Inorganic compounds might include heavy metals as well as elements in pure form or combined form with other elements.
- Inorganic compounds, like organic compounds, can be natural or synthetic. Natural inorganic compounds mostly occur in rocks in the form of minerals, whereas synthetic inorganic compounds can be produced in laboratories.
- Different physical and chemical properties of inorganic compounds are due to the type of elements present in them and the type of bonding.
- Inorganic compounds have higher boiling and melting points as a result of stronger bonding and complex structure.
- These have many applications in various industries as well as for the growth and development of different living organisms.
- Some of the common examples of inorganic compounds include carbon dioxide, sodium chloride, ammonia, etc.
13 Major Differences (Organic Compounds vs Inorganic Compounds)
| Characteristics | Organic Compounds | Inorganic Compounds |
| Definition | Organic compounds are chemical compounds composed of one or more carbon atoms bonded to other elements like hydrogen, oxygen, or nitrogen. | Inorganic compounds are compounds consisting of two or more elements, usually other than carbons, that are linked together in definite proportions and lack carbon-hydrogen linkages. |
| Composition | Organic compounds are composed of carbon, hydrogen, oxygen, and nitrogen. | Inorganic compounds are usually composed of elements other than carbon. |
| Occurrence | Organic compounds mostly occur in living organisms. | Inorganic compounds occur primarily on the Earth’s surface. |
| Chemical Bonding | Organic compounds consist of covalent bonding. | Inorganic compounds have different types of bonds. |
| Solubility | Most organic compounds do not dissolve in water due to their hydrophobicity. | Most inorganic compounds dissolve in water in the presence of ionic bonds. |
| Physical State | Most organic compounds exist in the liquid state, but they can exist in the solid and gaseous states. | Most inorganic compounds are solids. |
| Volatility | Most organic compounds are volatile and flammable. | Most inorganic compounds are not flammable and non-volatile. |
| Boiling and Melting Point | The boiling and melting point of organic compounds are usually low. | The boiling and melting point of inorganic compounds are relatively higher. |
| Nature | Organic compounds are biological and complex in nature. | Inorganic compounds aren’t as complex and are considered minerals. |
| Rate of Reaction | The rates of reactions involving organic compounds are slower. | The rates of reactions of inorganic compounds are comparatively faster. |
| Use | The most important use or purpose of organic compounds is to form biological structures in living beings. | Inorganic compounds act as a catalyst in living processes and are used for industrial applications. |
| Colour | Most organic compounds are colorless. | Most inorganic compounds are colored. |
| Examples | Some of the common examples of organic compounds include glucose, lysine, methane, acetone, etc. | Some of the common examples of inorganic compounds include carbon dioxide, sodium chloride, ammonia, etc. |
Examples of Organic Compounds
Methane
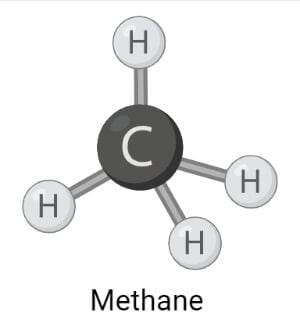
- Methane is the simplest organic compound that exists in the atmosphere in the gaseous form and is a by-product of different human activities.
- Methane is an aliphatic hydrocarbon consisting of a carbon atom compound singly-bonded to four hydrogen atoms. The molecular formula of methane is CH4.
- Methane is slightly soluble in water as it is weakly polar but is lighter than air with a specific gravity of 0.554.
- It is a highly flammable gas that burns with a pale, slightly luminous flame. Even though it is a stable gas, it is explosive when mixed with air at 5-14% by volume.
- Methane also has an important role as a fossil fuel, and it is produced as biogas for cooking purposes.
- It is a member of greenhouse gases known to be one of the prime gases involved in global warming and climate change.
Examples of Inorganic Compounds
Carbon Dioxide

- Carbon dioxide is an inorganic compound of carbon composed of a single carbon atom and two oxygen atoms bonded by covalent linkages.
- Carbon dioxide is the fourth most abundant component of air and is released by most living organisms during respiration.
- It is an inorganic compound as it lacks carbon-hydrogen bonds, and the carbon atom in the compound cannot form chains with other carbon atoms.
- In a carbon dioxide molecule, a single carbon atom is double-bonded to two separate oxygen atoms.
- Carbon dioxide is an essential gas for living systems as it is the source of carbon for photosynthesis and is released as a product during respiration.
- Carbon dioxide is an example of an inorganic compound that is incorporated into organic systems during the carbon cycle.
References and Sources
- Gautam AD, Pant M and Adhikari NR (2017). Comprehensive Chemistry Part 2. Heritage Publishers and Distributors. Kathmandu, Nepal.
- https://www.britannica.com/science/chemical-compound/Classification-of-compounds – 7%
- https://en.wikipedia.org/wiki/Organic_compounds – 5%
- https://www.aplustopper.com/properties-ionic-covalent-compounds/ – 3%
- https://vivadifferences.com/organic-vs-inorganic-compounds/ – 2%
- https://www.britannica.com/science/methane – 2%
- https://www.tiwariacademy.com/ncert-solutions/class-10/science/chapter-4/board-questions/ – 1%
- https://www.reference.com/science/examples-inorganic-compounds-dd6b6e6452c6a3a5 – 1%
- https://www.britannica.com/science/organic-compound – 1%
- https://scied.ucar.edu/learning-zone/how-climate-works/carbon-dioxide – 1%
- https://www.lifepersona.com/importance-of-carbon-in-living-beings-8-reasons – 1%
- https://scied.ucar.edu/learning-zone/how-climate-works/methane – 1%
