
Oxidation reactions are those in which atoms, molecules, or ions lose electrons, increasing the oxidation state.
Interesting Science Videos
Baeyer-Villiger oxidation
It involves the oxidation of aliphatic ketones to esters or their hydrolyzed products using hydrogen peroxide or organic peracids as oxidizing agents.
Baeyer villager oxidation is the oxidative transformation of an aldehyde and ketone into carboxylic ester and cyclic ketone to lactone using peroxy compounds such as hydrogen peroxide and peroxy acids in the presence of an acidic catalyst.
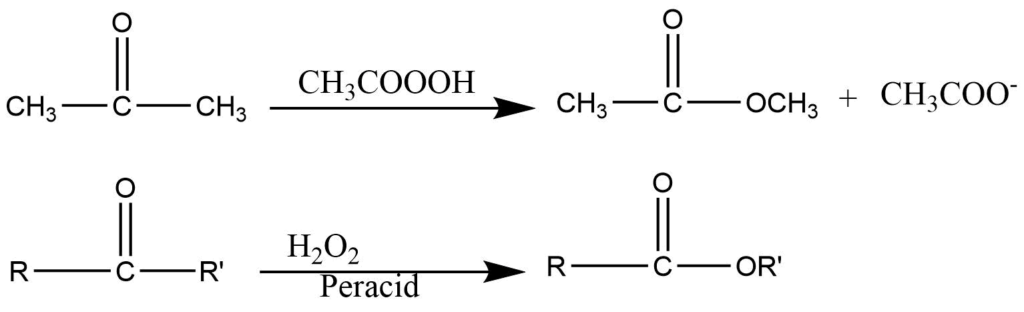
The baeyer villiger oxidation is characterized by an intramolecular aniontropic rearrangement in which the alkyl group with bonded electrons migrates from carbonyl carbon to electron-deficient oxygen.
Substitutes that can stabilize the positive charge migrate easily. The following is the migratory aptitude of different substituents are:
H > 3o-alkyl > cyclohexyl > 2o– alkyl > benzyl > aryl > 1o – alkyl > methyl.
Baeyer-Villiger oxidation is regioselective when the two ligands on the carbonyl carbon in the ketone are different. During this reaction the highly substituted alpha carbon migrates more preferentially.
Mechanism:
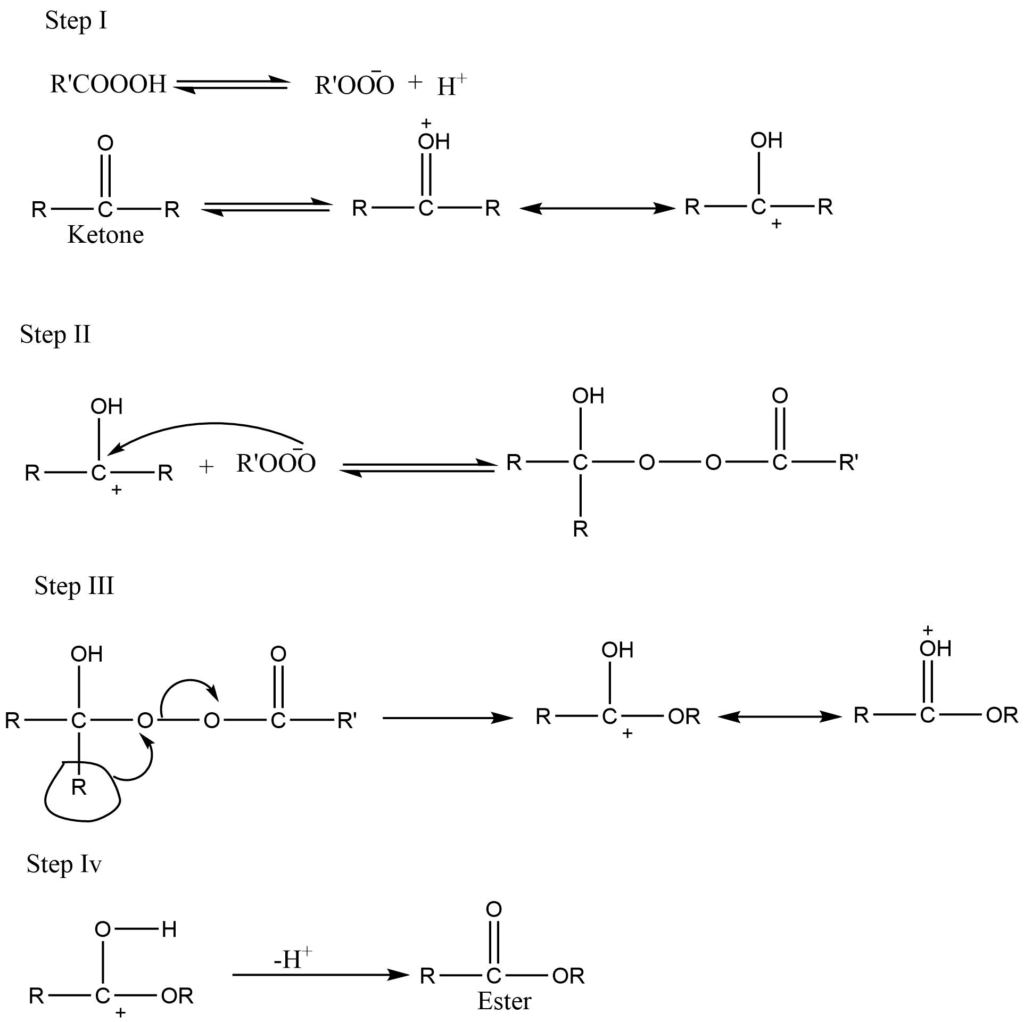
The reaction proceed throgh different steps they are as follow:
First step (I): The oxygen atom of the ketone is protonated in this step, and a resonance-stabilized carbonium ion is formed.
Second step (II): Nucleophilic attack of peroxy carboxylate ion at the carbonyl carbon.
Third Step (III): Migration of alkyl group and loss of RCOO– simultaneously.
Fourth step (IV): Loss of proton and formation of ester
Applications
- Preparation of aromatic ester from aromatic ketone

2. It gives a good yield of ester or acid. So it is used to prepare esters and carboxylic acids.
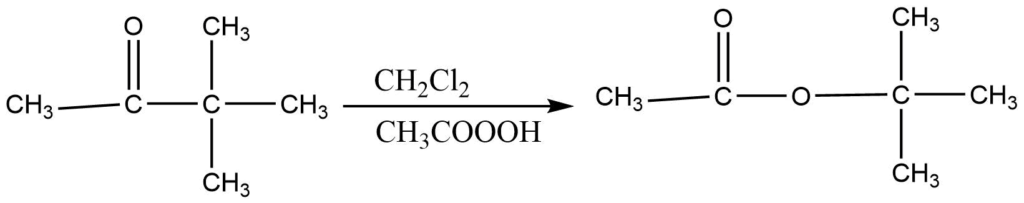
Oppenauer oxidation
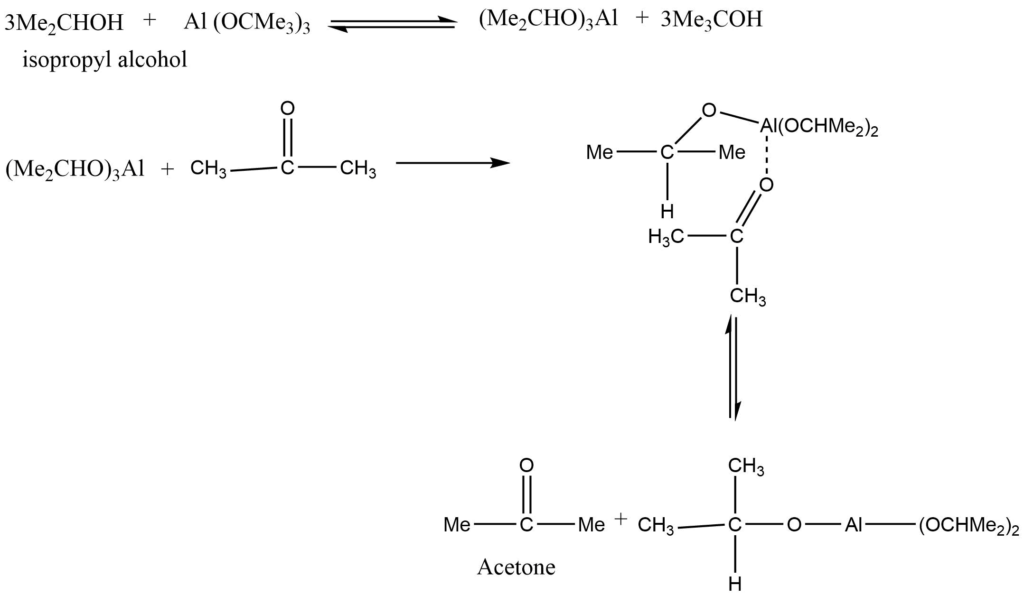
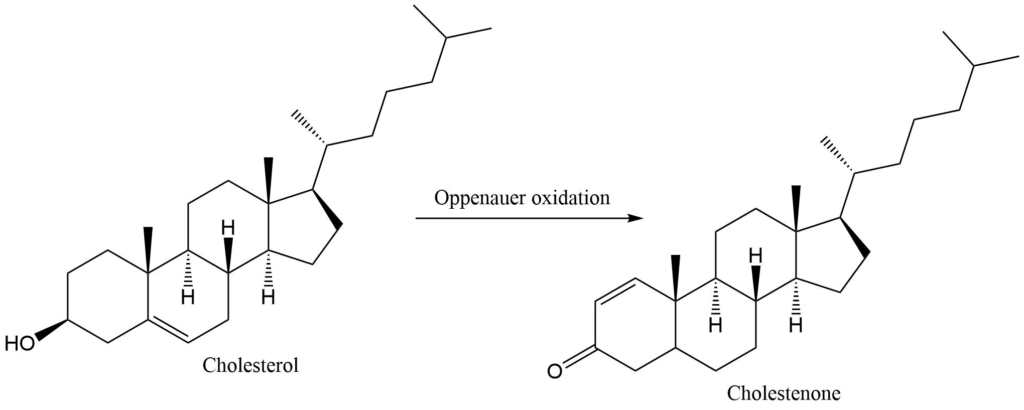
Oppenauer oxidation is a chemoselective oxidation reaction that involves the oxidation of secondary alcohols to ketones in presence of aluminium tertiary butoxide in benzene or toluene solution. It is the oxidation of a secondary alcohol to the corresponding ketone catalyzed by aluminium alkoxide. A large excess of the ketone is used to proceed with the reaction in the desired direction. It is the reverse of Meerwein-Ponndrof-Verley reduction.

Mechanism
In the first step, alcohol forms a complex with aluminium isopropoxide. This complex on reaction with a ketone gives a six-membered transition complex. The aluminium-catalyzed hydride shift converts the alcohol’s alpha-carbon to the carbonyl carbon. The desired product is formed by the hydride shift.
Applications
- Since it is specific for alcohols, it is used to oxidize alcohol in the presence of another oxidizable functional group (olefinic bond, phenolic group).
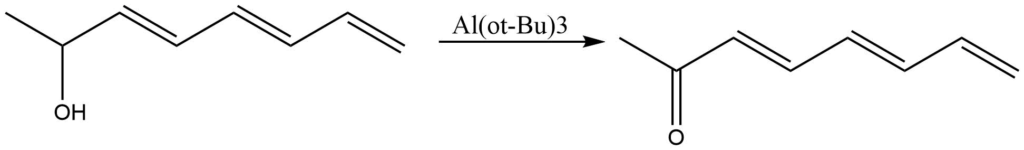
2. It is used for oxidation of formates to a carbonyl group.
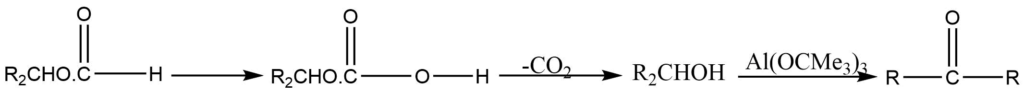
3. oxidation of cholesterol to cholestenone
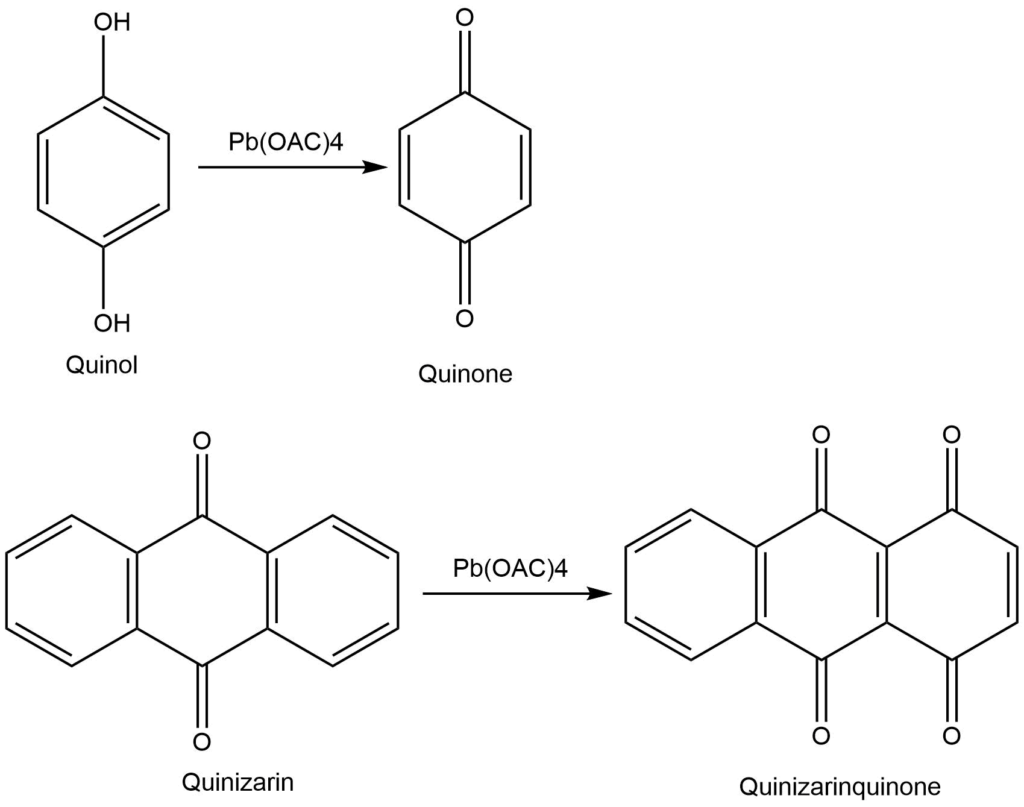
Lead tetraacetate oxidation
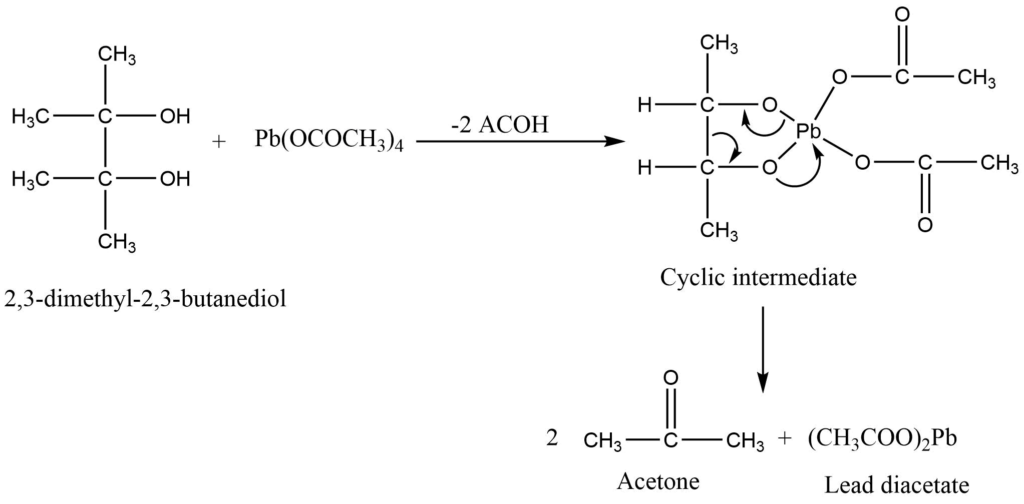
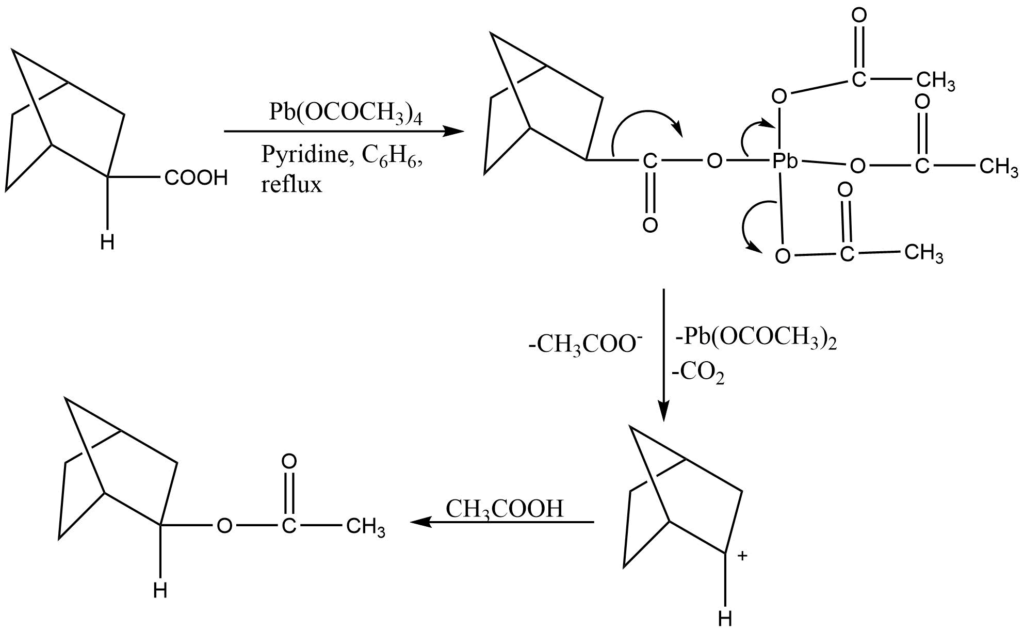
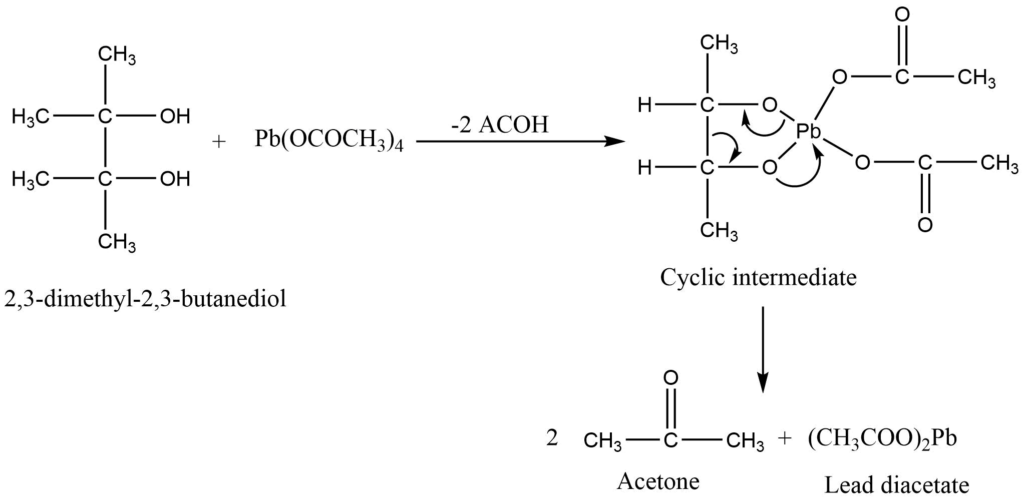
Lead tetraacetate is a commonly used reagent for the cleavage of glycol and the preparation of carbonyl compounds. The reaction is carried out in the presence of either an aprotic solvent (nitrobenzene, benzene) or a protic solvent (acetic acid). The most frequently oxidizable groups are hydroquinones, 1,2 diols, α-hydroxy acids, and α-ketoacids.
Mechanism of Oxidation of 1,2 diol
Lead tetraacetate attacks on diols to give the cyclic transition state which is readily cleaved to give the product.
Application
1.Oxidation of alcohols
Lead tetraacetate in refluxing benzene, and hexane is a good reagent for the oxidation of primary and secondary alcohols to the corresponding aldehyde or ketone.

2. Oxidative degradation of carboxylic acid
3. Oxidation of diols
Chromic acid oxidation
Chromic acid is widely used for the oxidation of alcohols, aldehyde, and carbon-hydrogen bonds in hydrocarbons. Usually, chromic acid is made in situ through the addition of acid to the source of chromium. Sodium dichromate, potassium dichromate, sodium chromate, potassium chromate, and chromium trioxide are common sources of chromium and are used as oxidizing agents.
a. oxidation of alcohols
The solution of alcohol in acetone on treatment with jones reagent (i.e., A solution of chromic acid and sulphuric acid in water), gives ketone, without disturbing double bonds or triple bonds that may be present.
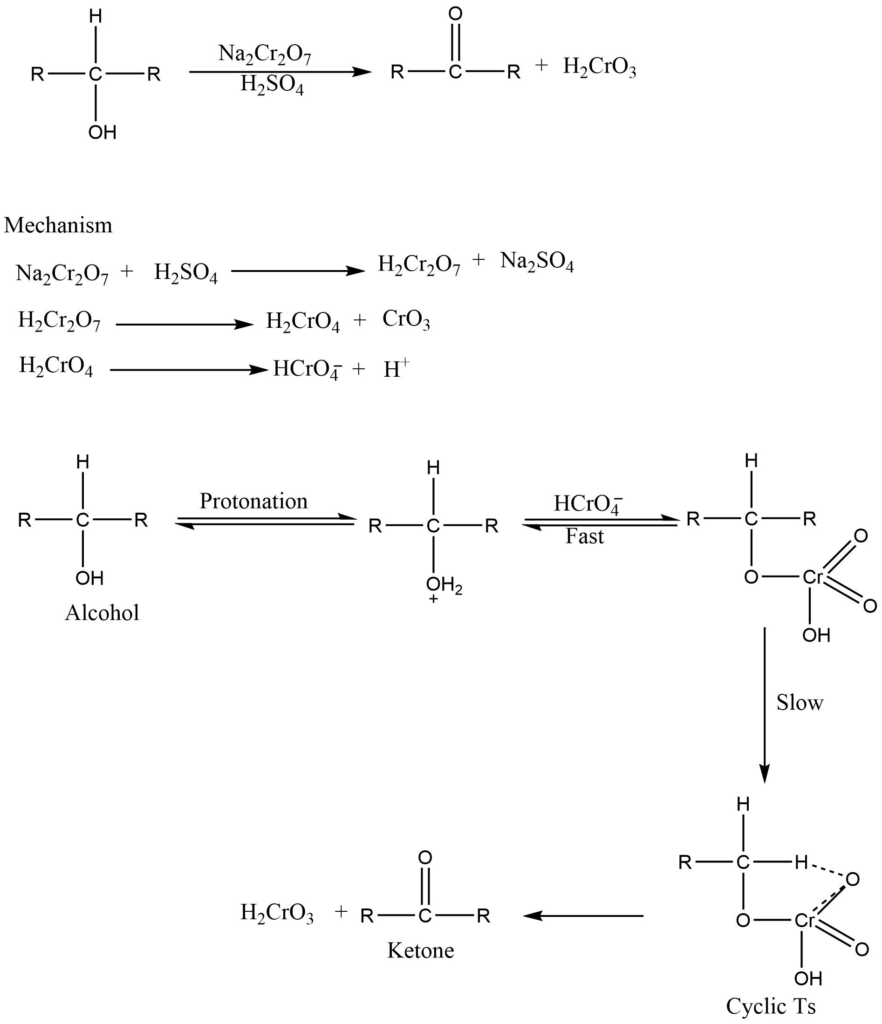
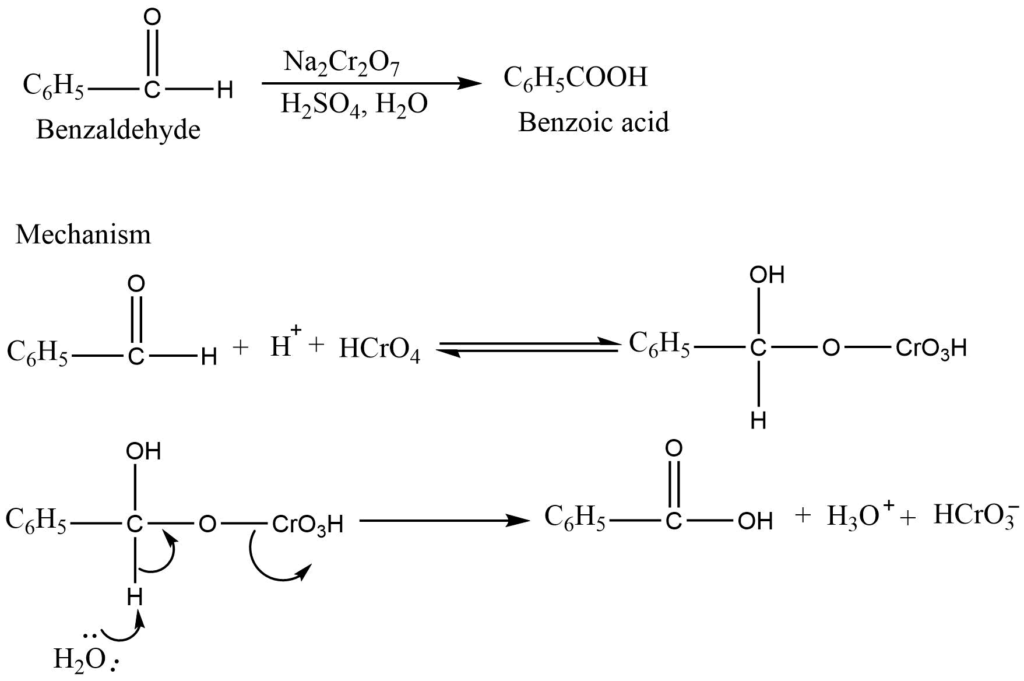
b. Oxidation of aldehyde
The reaction of aldehyde and ketone with an aqueous and acidic solution of sodium dichromate produces carboxylic acid.
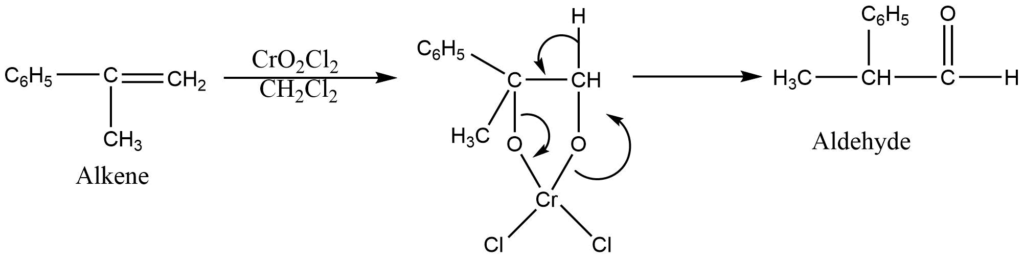
c. oxidation of carbon-carbon double bond
Chromic acid in partially aqueous medium favours oxidative cleavege of carbon-carbon double bond.
Permanganate Oxidation
Potassium permanganate is widely used for the oxidation of alcohols, aldehyde, carbon-hydrogen bonds.
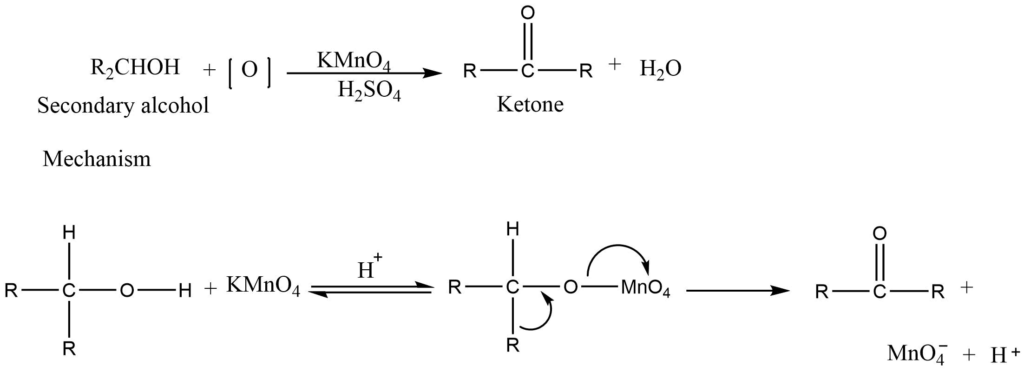
a. oxidation of primary alcohol
The primary alcohol is oxidized first into aldehyde and then into a carboxylic acid. Secondary alcohol is oxidized to a ketone using an acidified solution of permanganate.
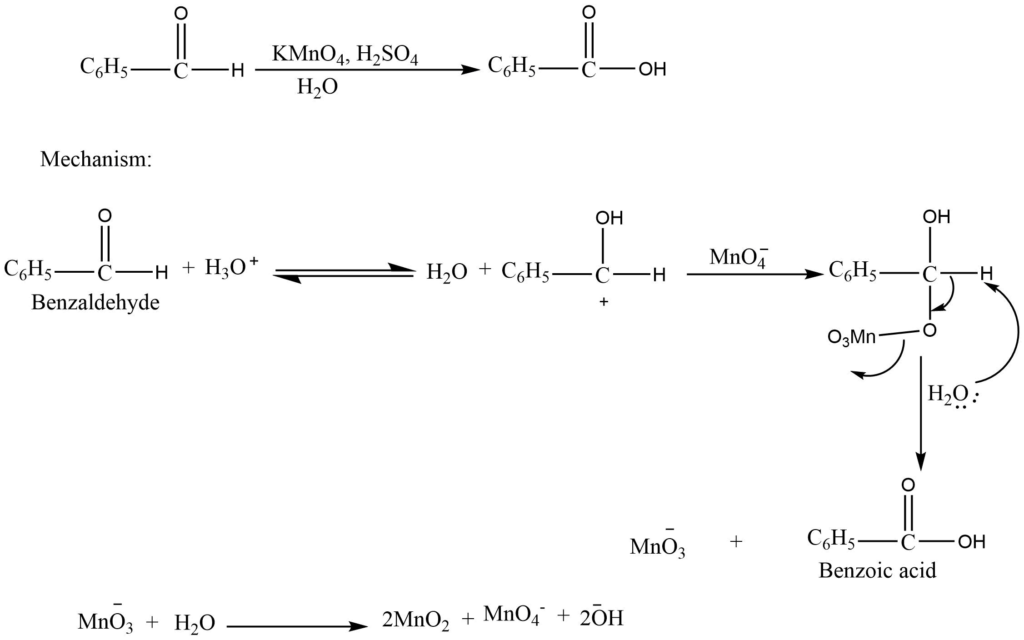
b. Oxidation of aldehyde
The aldehyde can be converted into carboxylic acid by using the acidified or basic solution of potassium permanganate.
c. Oxidation of carbon-carbon double bonds
Alkene reacts with a cold dilute solution of potassium permanganate to produce 1,2-diols i.e., glycol.
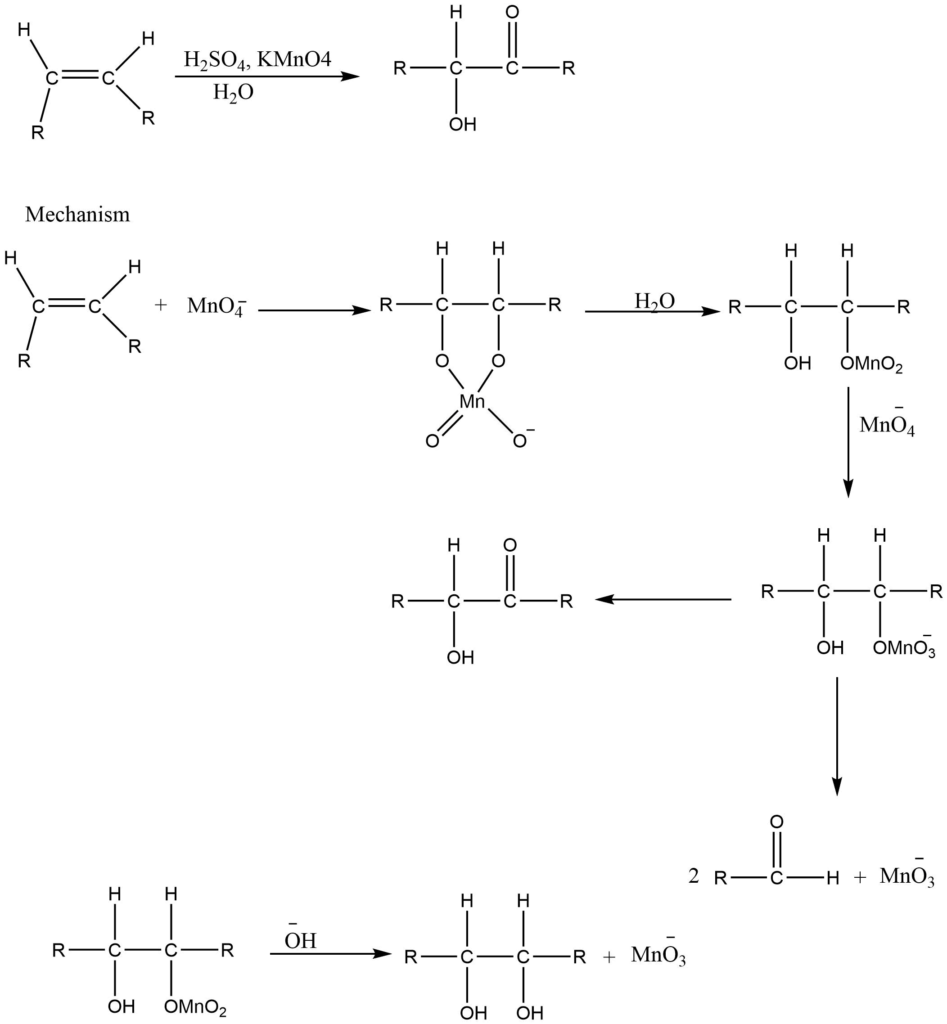
d. Oxidation of carbon-hydrogen bonds in hydrocarbon
In presence of an alkaline solution of potassium permanganate, the side chain of the alkyl benzene is converted into corresponding benzoic acid.
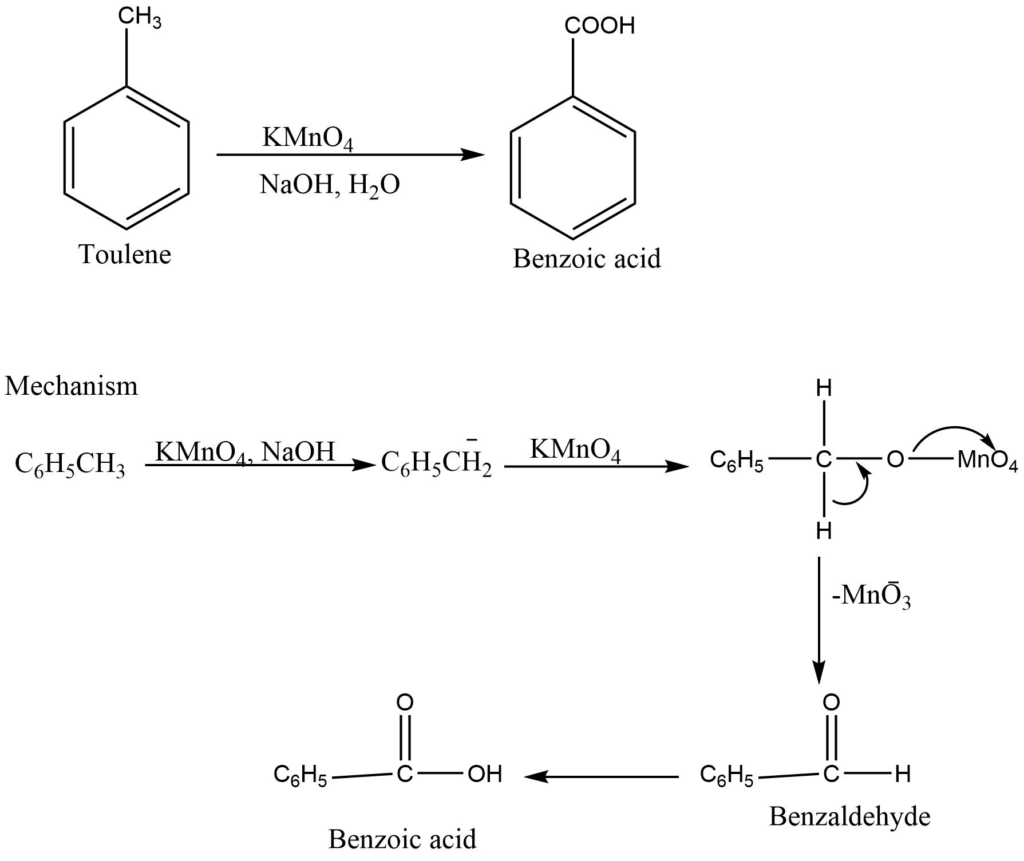
Peracid oxidation
Peracids have been extensively used for the selective oxidation of carbon-carbon double bonds. Ketones on reaction with peracid produce esters.
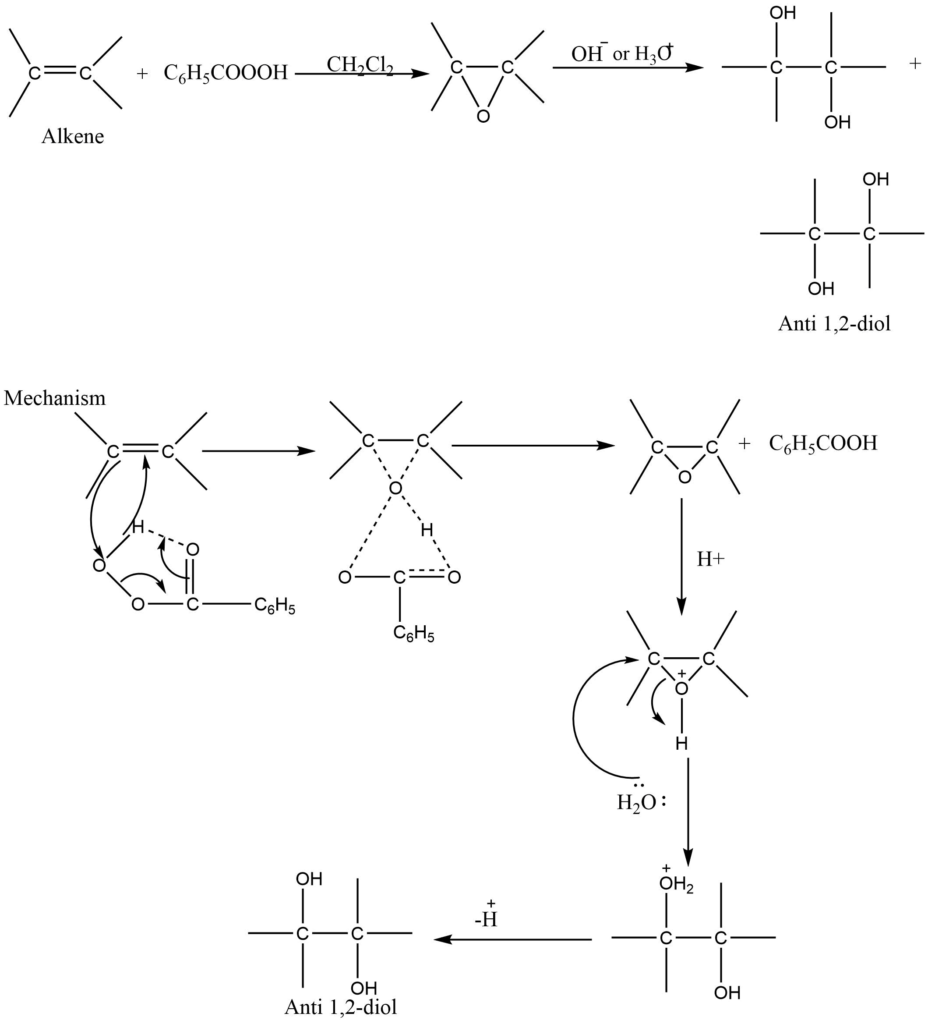
Application
This is mainly used for the selective preparation of trans diol.
Hydroboronation oxidation
The addition of borane to the carbon-carbon double bond by the action of diborane to form alkyl borane is known as hydroboration. Hydroboration involves the simultaneous addition of hydrogen and boron to alkene through the Pi complex to form a four-centered transition state in which boron is attached to the least substituted carbon of the double bond. The trialkyl borane formed from the hydroboration reaction is oxidized to the alcohols by hydrogen peroxide in aqueous sodium hydroxide.
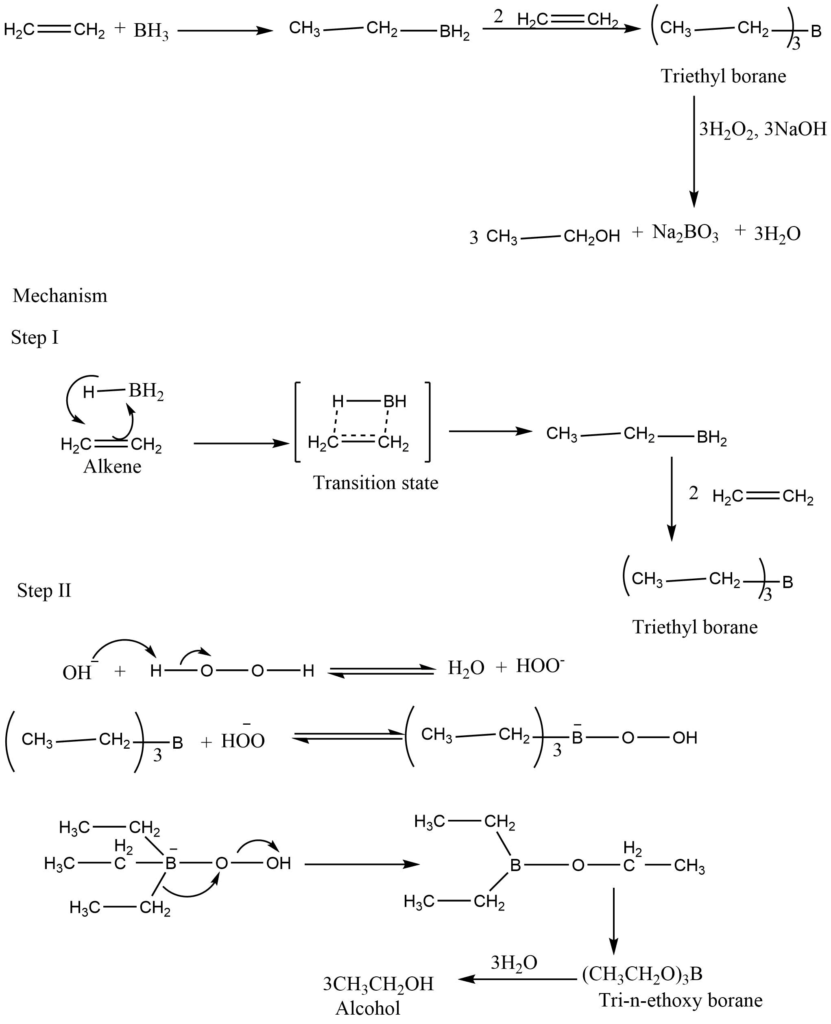
Application
It is mainly used for the alcohols from an alkene. It is stereoselective .
For more information watch this video
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Solomons, T. W. Graham. (2011). Organic chemistry. Hoboken, NJ :Wiley,
- https://byjus.com/chemistry/oppenauer-oxidation/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols/Reactivity_of_Alcohols/The_Oxidation_of_Alcohols/Oxidation_by_Chromic_Acid
- https://juniperpublishers.com/omcij/pdf/OMCIJ.MS.ID.555788.pdf
- https://www.chem.ucalgary.ca/courses/350/Carey5th/Ch17/ch17-3-5-2.html
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Oxidation_and_Reduction_Reactions/Oxidation_of_Organic_Molecules_by_KMnO4
- https://www.masterorganicchemistry.com/reaction-guide/hydroboration-of-alkenes/
