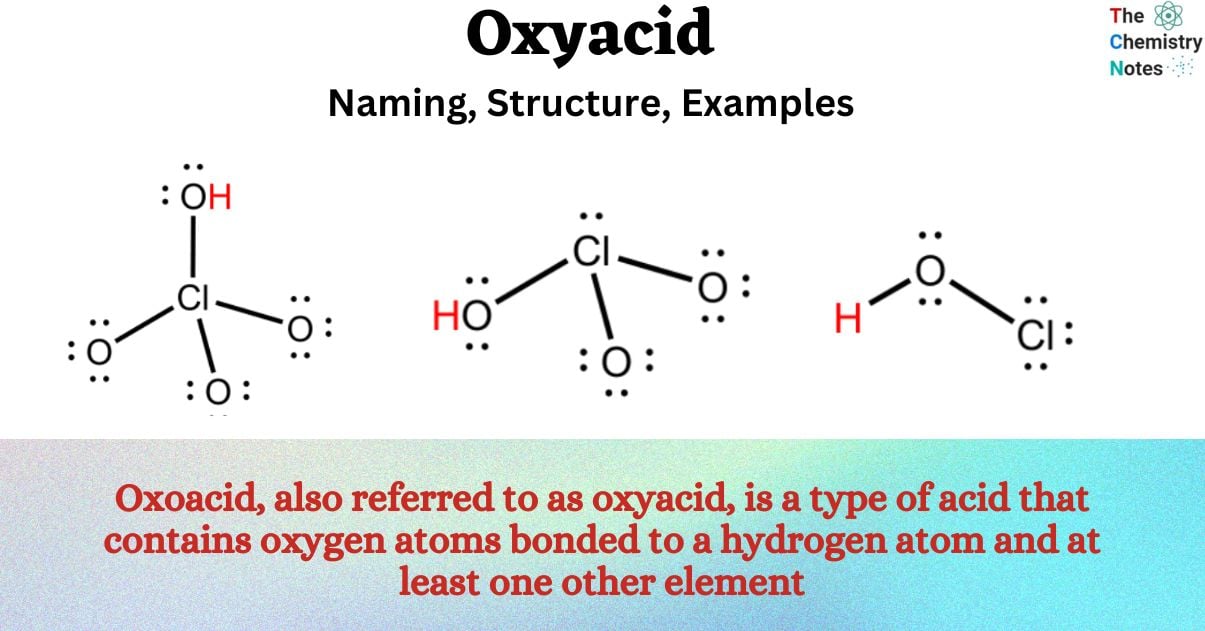
An oxyacid refers to a type of acid that consists of a hydrogen atom bonded to an oxygen atom, along with the presence of at least one additional element. When an oxyacid is introduced to water, it undergoes dissociation, resulting in the formation of the H+ cation and the corresponding anion of the acid. An oxyacid is a type of acid that follows the general structure of X-O-H.
Interesting Science Videos
What are Oxyacids?
Oxoacid, also referred to as oxyacid, is a type of acid that contains oxygen atoms bonded to a hydrogen atom and at least one other element. These compounds are characterized by their ability to donate protons (H+)
Sulfuric acid (H2SO4), phosphoric acid (H3PO4), and nitric acid (HNO3) are classified as oxyacids.
Naming of Oxyacids
The process of naming oxyacids involves assigning appropriate names to these compounds based on their chemical composition and structure. Oxyacids are a type of acid that contains oxygen, hydrogen, and another element.
An oxyacid is a type of compound that contains a chemical linkage between atom A and the hydroxyl group (OH). This linkage is typically referred to as A-O-H. In this chemical arrangement, the hydrogen atom is paired with the oxygen atom. Non-metal A is known for its characteristic of not possessing metallic properties.
- One notable attribute of non-metals is that their oxides tend to exhibit acidic properties. Therefore, the term “oxyacid” is derived. When naming a new oxyacid, it is important to consider the parent oxyacids.
- Oxyacids are typically named using the suffixes “ic” or “ous” to indicate their composition and oxidation state. In the case of oxyacids with a higher oxidation state, they are typically named using the suffix “ic”.
- When an element is in a lower state of oxidation, it is common to add the suffix “ous” to the resulting compound. However, it is important to note that the naming of oxyacids is not solely determined by the higher or lower state of oxidation. The oxidation state of an element can undergo changes when oxygen is either added or removed from a compound. In addition, altering the water content of an oxyacid has the potential to induce modifications in its structure and composition.
- Parent oxyacids are characterized by a higher degree of oxidation. Here are a few examples:
- In the case of the Boron (B) family, the chemical compound that is formed is known as H3BO3, which is commonly referred to as Boric acid.
- Nitrogen (N) undergoes a chemical reaction to form HNO3, which is commonly known as nitric acid.
- When silicon (Si) combines with hydrogen (H) and oxygen (O), it forms a compound known as silicic acid. The chemical formula for silicic acid is H4SiO4.
- When phosphorus (P) combines with hydrogen (H) and oxygen (O), it forms a compound known as phosphoric acid, which is represented by the chemical formula H3PO4.
The aforementioned examples are presented in the parent oxyacid format.
- When the oxygen content of an oxyacid is reduced, the oxidation state of the compound also decreases. Consequently, the element undergoes a reduction in its oxidation state, resulting in the formation of a lower state known as the “ous” form. By reducing the oxygen content by one, the resulting form of the element will be the hypo form, also known as the hypo of the -ous form. Following that, the Meta form of the “ous” form can be obtained by intramolecularly reducing H2O. When water molecules are reduced, they undergo a chemical transformation resulting in the formation of the pyro form of an oxyacid in its “ous” form.
- The Meta form of an oxyacid is obtained by removing an intramolecular H2O molecule from the parent oxyacid, resulting in a transformation from the “ic” form to the Meta form. When water molecules are reduced, they can form the Pyro form, also known as the “di” form or the “ic” form.
- By eliminating oxygen from the Pyro variant of oxyacid, the resulting compound will be the Hypo variant of the “ic” form. When oxygen is introduced in the pyro form, it undergoes a transformation to the di form of the per “ic” form.
- When oxygen is added directly to the parent oxyacid, it results in the formation of a perform or per oxy form of the “ic” acid. This particular type of oxyacids is characterized by its monoprotic or single-acidic nature.
Phosphorus oxyacids
Phosphorus oxyacids are a group of chemical compounds that contain phosphorus, oxygen, and hydrogen atoms. These acids are formed when phosphorus reacts with oxygen and water.
Phosphorus is a fundamental component in the creation of oxoacids.
- The primary representatives of oxoacids of phosphorus are phosphorous acid (H3PO3) and orthophosphoric acid (H3PO4).
- In the context of oxoacids of phosphorus, it is noteworthy that the phosphorus atom is encompassed by other participating atoms on all four sides, resulting in the formation of bonds. The oxoacids of phosphorus exhibit three distinct types of covalent bonds.
- The P – P or P – H links are commonly found in conjunction with the P-OH bond and the P=O bond. The oxidation states of these acids undergo a shift from high to low due to the fact that the oxidation number of the phosphorus atom does not exceed 5.
The oxoacids of phosphorus exhibit a range of acidic strengths, which can be ordered as follows:
H3PO2>H3PO3 > H3PO4
Phosphorus acid (H3PO2)
It is a compound that is commonly obtained as an intermediate product during various chemical reactions involving phosphorus. This substance possesses diprotic acid properties, which means that it has the ability to dissociate into ions when it comes into contact with water. The production of this particular oxoacid occurs through a process known as hydroxylation.
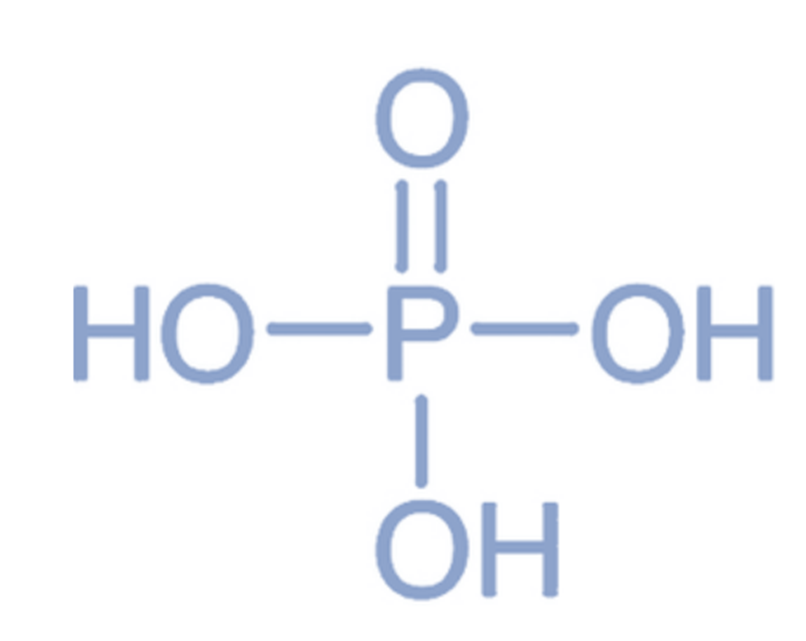
Phosphoric Acid (H3PO4)
It is a compound composed of three hydrogen molecules and one phosphorus atom. Due to its composition, it is commonly known as atricrotic acid. The acid in question is formed through the chemical reaction between sulphuric acid and solid tricalcium phosphate.
Meta phosphoric acid (HPO3)
It is an oxoacid that does not exist in a monomeric form. To obtain metaphosphoric acid, the compound H3PO4 is subjected to a heating process at approximately 850°C. When protons undergo disintegration, they undergo a breakdown process that results in the production of anions in the form of metaphosphates.
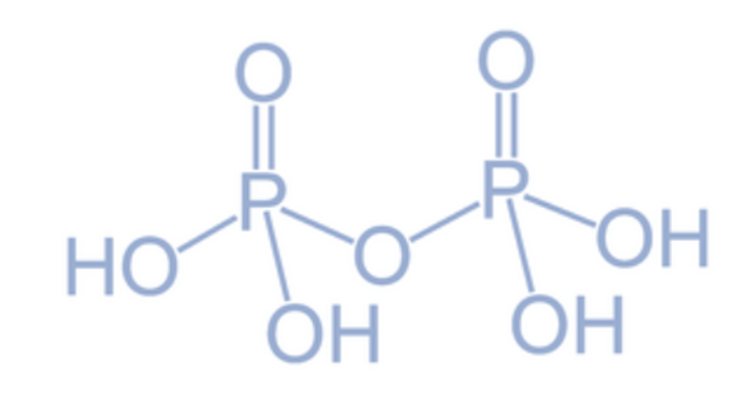
Pyrophosphoric acid (H4P2O7)
Pyrophosphoric acid, also known as H4P2O7, is classified as a tetrabasic variant of oxoacids of phosphorus. When phosphoric acid (H3PO4) is subjected to thermal treatment at approximately 250°C, it undergoes a chemical transformation and forms pyrophosphoric acid.
Phosphorus acid, also known as hypophosphoric acid, can be synthesized through the oxidation of red-hot phosphorus. The reaction employs the utilization of sodium chlorite.
Oxyacids of Chlorine
The oxoacids of chlorine are a group of compounds that contain chlorine, oxygen, and hydrogen atoms. These compounds are formed when chlorine reacts with water or other substances that contain oxygen.
There are four main types of oxoacids that can be derived from chlorine. The names of these compounds are hypochlorous acid, chlorous acid, perchloric acid, and chloric acid.
- The production of glucose and chlorine is closely linked to various industrial processes, thereby serving a specific purpose. These acids play a crucial role as essential components in the field of modern medicinal chemistry.
- Hydrolytic disproportion refers to a chemical reaction that occurs when gaseous chlorine is introduced into a water medium. The reaction at hand is known to be reversible and results in the formation of hypochlorous acid also referred to as HClO.
- Chlorous acid, a prominent oxoacid of chlorine, is widely found in nature due to its abundance. It is typically synthesized through the oxidation process of lead chloride or barium. The addition of diluted sulphuric acid serves to facilitate the reaction.
- The oxidation of barium chlorate is commonly performed using concentrated sulphuric acid. The chemical reaction results in the formation of chloric acid. The chemical formula for the compound in question is HClO3. By choosing to perform the oxidation of barium perchlorate instead of barium chlorate, we can expect the resulting product to be HOClO3, commonly known as perchloric acid. This outcome can be achieved by following the same procedure as before.
- All of the oxoacids of chlorine readily dissolve when added to water.
| Name | Formula |
|---|---|
| Perchloric acid | HClO4 |
| Chloric acid | HClO3 |
| Chlorous acid | HClO2 |
| Hypochlorous acid | HClO |
Oxyacid of Halogens
The oxoacids of halogens refer to a group of compounds that are formed when halogens, such as chlorine, bromine, or iodine, combine with oxygen and hydrogen.
The aforementioned discussion pertains to the family of chlorine oxoacids, which falls under the category of oxoacids of halogens. The halogens are a group of elements that are located in group 17 of the classical periodic table. Halogens, such as bromine, iodine, chlorine, and fluorine, serve as prime examples within the periodic table.
Bromine and iodine are elements that have the ability to form three distinct types of oxoacids. The presence of sp3 hybridization can be observed in the central atom of halogen oxoacids. The X=O bonds found in halogen oxoacids are known to exhibit a distinctive characteristic known as d pi-pi bonding. The level of acidity in a compound is directly influenced by the oxidation state of the halogen element present.
Video on Naming of Oxyacids
References
- https://unacademy.com/content/upsc/study-material/chemistry/a-simple-note-on-oxyacids/
- https://www.britannica.com/science/oxyacid/Formation-of-sulfate-salts
- Helmenstine, Anne Marie, Ph.D. “What Is an Oxyacid in Chemistry?” ThoughtCo, Aug. 25, 2020, thoughtco.com/definition-of-oxyacid-605461.
- https://dornshuld.chemistry.msstate.edu/books/chemistry/oxyacids.html
- https://www.alonsoformula.com/inorganic/oxacidos.htm
- https://www.geeksforgeeks.org/oxoacids-of-phosphorus/
- https://collegedunia.com/exams/oxyacids-and-ammonia-definition-structure-properties-and-uses-chemistry-articleid-4359

