Penicillin is a kind of antibiotic that is often used to treat gram-positive and gram-negative bacterial infections. Due to the presence of a beta-lactam ring in their structure, these medications are also known as beta-lactam antibiotics.
In 1928, Sir Alexander Fleming, a Scottish researcher, discovered Penicillin by accident while examining the properties of Staphylococci. The Penicillium mold produced penicillin, one of the first and still most extensively used antibiotics. Penicillin is one of the oldest and most extensively used antibiotics. Furthermore, penicillin is regarded as one of the safest and most effective antibacterial agents.
Interesting Science Videos
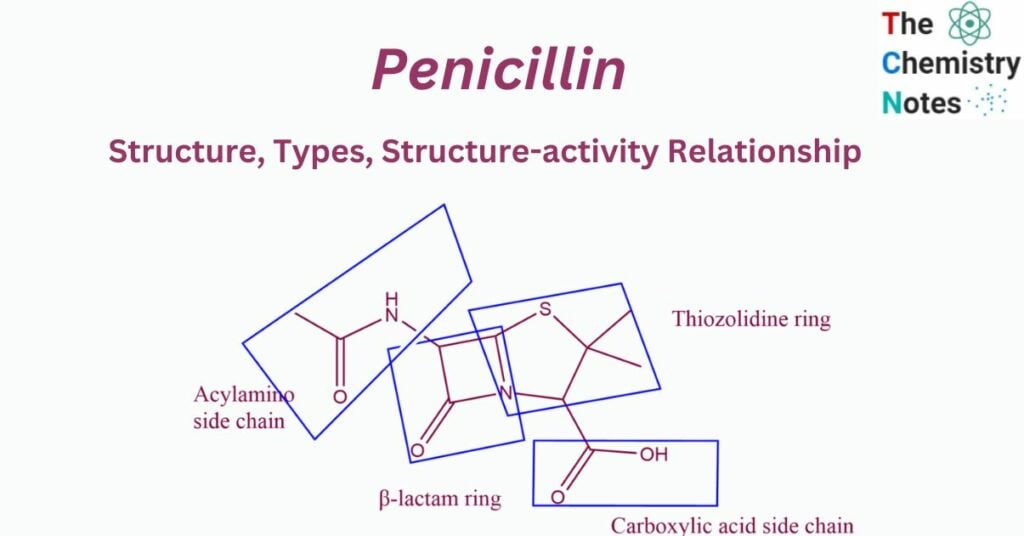
Structure of penicillin
Beta-lactam antibiotics, which include penicillins and cephalosporins, are distinguished by three essential structural requirements:
- The fused beta-lactam & Thiazolidine ring structure.
- a free carboxyl acid group.
- One or more substituted amino acid side chains.
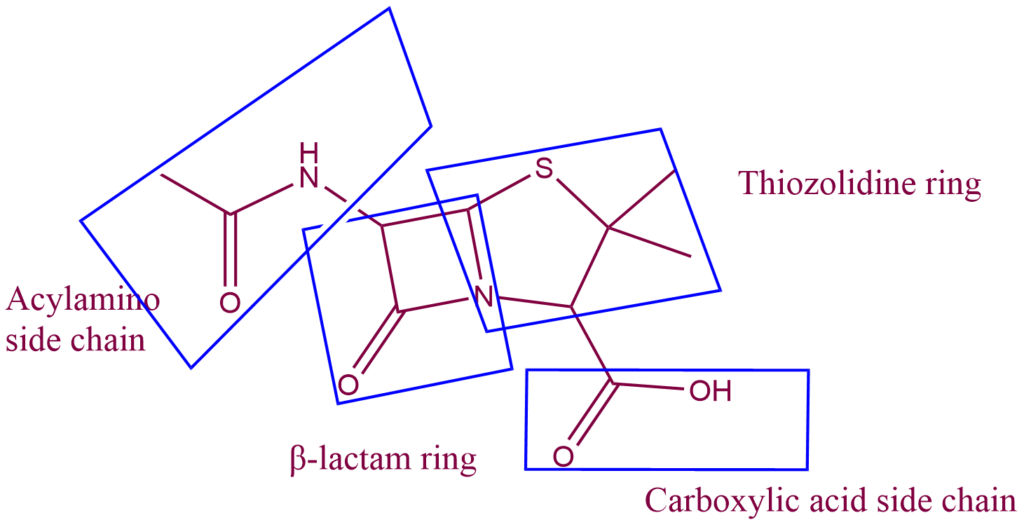
All penicillins have the same basic structure: a thiazolidine ring linked with a beta-lactam ring, resulting in a fundamental nucleus (also known as 6-amino penicillanic acid) that is essential for their antibacterial activity. By changing the composition of the side chains connected to this nucleus, a variety of semisynthetic penicillins are created.
Strong electron-attracting groups linked to the amino acid side chain can avoid an acid attack. A bulky group connected to an amino acid side chain causes steric hindrance, which interferes with the enzyme attachment that can potentially deactivate penicillins (for example, methicillin). The higher polarity of the molecule, as with ampicillin or carbenicillin, results in higher activity against Gram-negative bacteria.
Mechanism of action
Penicillin works by stopping bacteria from retaining their cell walls. The bacterial cell wall is an important component consisting of peptidoglycan, which helps the cells maintain their form.
Bacteria cannot survive without their cell wall, resulting in cell bursting (lysis) and cell death. Penicillins are categorized as bactericidal antibiotics since they directly destroy bacteria. Penicillins can kill a variety of bacteria but are more effective against gram-positive bacteria.
Penicillin and other beta-lactam antibiotics have a four-membered beta-lactam ring. By binding the beta-lactam ring to D-transpeptidase, penicillin kills bacteria by preventing cross-linking and reducing cell wall production. Without a cell wall, a bacterial cell is exposed to outside water and molecular pressures, causing it to die quickly. Penicillin therapy kills bacteria but does not affect human cells since they lack a cell wall.
Nomenclature
Nomenclature of penicillins is done to different systems:
Chemical abstract system(CAS):
The penicillins are numbered starting with the “S” atom in accordance with this system.
The compound is known as 6-acylamino-2,2-dimethyl-3-corboxylic acid and has the sulfur atom in the number one position and the “N” atom in the number four position.
United States Pharmacopoeia (USP system):
The CA scheme is reversed in the USP system for designating penicillins. This system gives the nitrogen atom the first position and the S atom the fourth position, resulting in a compound known as 4-thia-1-azabicyclo heptone.
Types of penicillin
Classification on the basis of source
Natural
- Penicillin-G
- Penicillin-V
Semi-synthetic
- Oxacillin
- Cloxacillin
- Dicloxacillin
- Methicillin
- Nafcillin
- Ampicillin
- Amoxycillin
- Carbenicillin
- Piperacillin
Route of administration
Oral
- Ampicillin
- Amoxycillin
- Penicillin-V
- Oxacillin
- Cloxacillin
- Dicloxacillin
Parenteral
- Penicillin-G
- Methicillin
- Nafcillin
- Carbenicillin
- Piperacillin
Spectrum of activity
Narrow spectrum
- Penicillin-G
- Penicillin-V
Broad spectrum
- Ampicillin
- Amoxycillin
Intermediate spectrum
- Methicillin
- Oxacillin
- Nafcillin
- Dicloxacillin
Extended-spectrum
- Carbenicillin
- Ticarcillin
- Piperacillin
- Mezlocillin
Resistance to enzyme
Resistance to ß-lactamase
- Methicillin
- Nafcillin
- Oxacillin
- Cloxacillin
- Dicloxacillin
Non -resistance to ß-lactamase
- Penicillin-G
- Penicillin-V
- Ampicillin
- Amoxycillin
- Carbenicillin
Resistance to acid
Acid stable
- Penicillin-V
- Ampicillin
- Amoxycillin
- Oxacillin
- Cloxacillin
- Dicloxacillin
Acid unstable
- Penicillin-G
- Methicillin
- Nafcillin
- Carbenicillin
- Piperacillin
- Ticarcillin
Structure-activity relationship
Position 1 — Oxidising the sulfur atom of the Thiazolidine ring to a sulfone or sulfoxide enhances acid stability but decreases the activity of the agent.
Position 2 — No substitutes are permitted at this position; any change will result in a lower activity. The methyl groups are required.
Position 3 — Thiazolidine’s carboxylic acid is necessary for action. If it is converted to an alcohol or ester, its activity decreases.
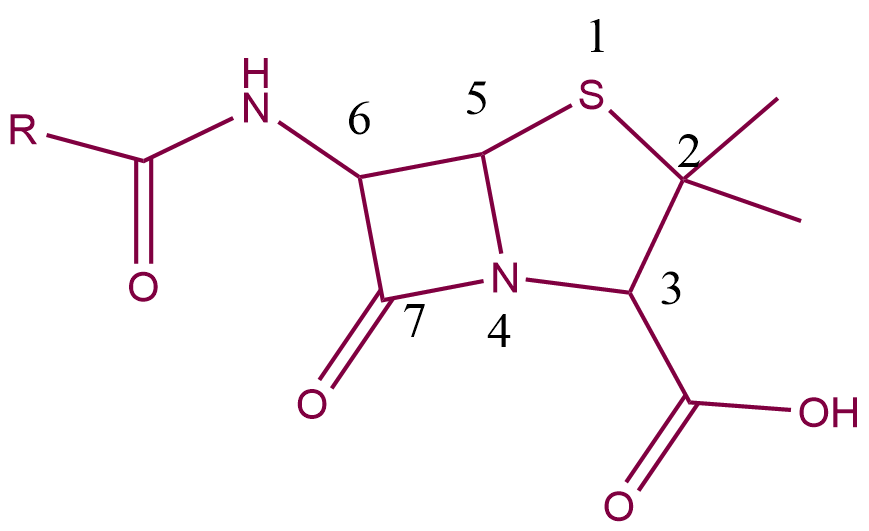
Position 4 — Nitrogen is required.
Position 5 — No substitutes are permitted.
Position 6 — Substitutions on the amide’s side chain are permitted.
Position-7 – Carbonyl on the Beta-lactam ring is required in position 7.
Because this substitution makes the amide oxygen less nucleophilic, an electron-withdrawing group added at this position improves acid stability.
The addition of a bulky group near the ring strengthens the compound’s resistance to Beta-lactamases.
The Beta-lactam ring is protected by steric hindrance.
Uses of Penicillin
- skin infection
- dental infection
- ear infection
- an infection of the nose, throat, or lungs
- urinary tract infection
- other bacterial infections
References
- https://www.ncbi.nlm.nih.gov/books/NBK554560/#:~:text=Penicillin%20is%20a%20medication%20used,valuable%20agent%20in%20treating%20infection.
- https://www.britannica.com/science/penicillin.
- https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/VetInfo/VetAntibioResSen/LADDL/AntimicrobialClasses/beta-lactams/penicillins/penicillin.pdf.
- https://www.news-medical.net/health/What-is-Penicillin.aspx
