The periodic Table with atomic mass stands as a foundational cornerstone in the field of chemistry, presenting a systematic structure for organizing and comprehending the fundamental constituents of matter. This table arranges elements by their atomic numbers, which denote the number of protons within their nuclei. This systematic arrangement reveals recurring patterns, grouping elements with similar chemical properties into columns or groups. By integrating information on atomic masses, this table emerges as a potent information, enhancing our understanding of element composition and behavior and underscoring its pivotal role in delving into the multifaceted realm of chemistry.
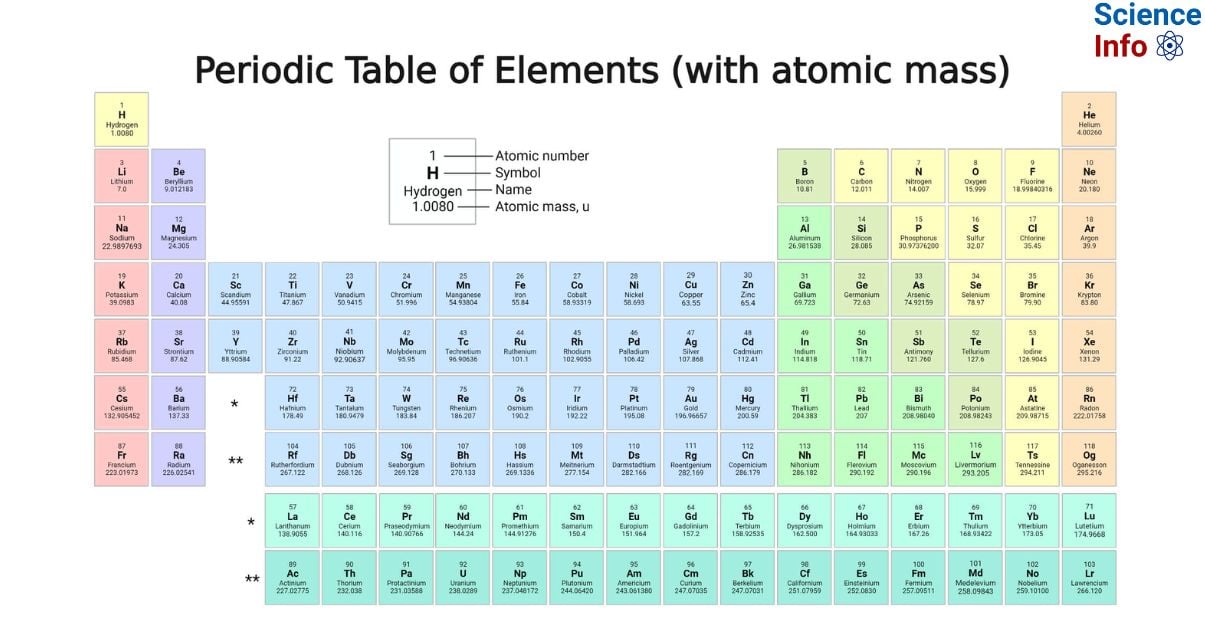
The atomic mass of an element is defined as the total mass of a single atom of that specific element and is measured in unified atomic mass units (u). One unified atomic mass unit is equivalent to the mass of one-twelfth of a carbon-12 atom at rest. Given that protons and neutrons contribute significantly to the atom’s mass, the atomic mass of an element is nearly identical to its mass number. Therefore, the atomic mass is determined using unified atomic mass units and reflects the combined mass of protons and neutrons in an atom.
Indeed, elements with atomic numbers greater than 104 fall into the category of superheavy elements. As the atomic number increases, indicating more protons in the nucleus, the size of the nucleus grows, leading to increased instability in general. The larger the nucleus, the more challenging it becomes for the strong nuclear force to counteract the repulsive forces between positively charged protons.
This increased instability often results in shorter half-lives for the isotopes of superheavy elements, making them difficult to study and observe for an extended period. Researchers face considerable challenges in synthesizing and studying these elements due to their fleeting existence and the complex nuclear reactions involved in their creation. The study of superheavy elements contributes valuable insights into nuclear physics and the limits of stability within the periodic table.
Interesting Science Videos
Elements in Periodic Table with Atomic Mass
| Element | Atomic Number | Symbol | Atomic Mass |
| Hydrogen | 1 | H | 1.008 |
| Helium | 2 | He | 4.0026 |
| Lithium | 3 | Li | 6.94 |
| Beryllium | 4 | Be | 9.0122 |
| Boron | 5 | B | 10.81 |
| Carbon | 6 | C | 12.011 |
| Nitrogen | 7 | N | 14.007 |
| Oxygen | 8 | O | 15.999 |
| Fluorine | 9 | F | 18.998 |
| Neon | 10 | Ne | 20.180 |
| Sodium | 11 | Na | 22.990 |
| Magnesium | 12 | Mg | 24.305 |
| Aluminum | 13 | Al | 26.982 |
| Silicon | 14 | Si | 28.085 |
| Phosphorus | 15 | P | 30.974 |
| Sulfur | 16 | S | 32.06 |
| Chlorine | 17 | Cl | 35.45 |
| Argon | 18 | Ar | 39.948 |
| Potassium | 19 | K | 39.098 |
| Calcium | 20 | Ca | 40.078 |
| Scandium | 21 | Sc | 44.956 |
| Titanium | 22 | Ti | 47.867 |
| Vanadium | 23 | V | 50.942 |
| Chromium | 24 | Cr | 51.996 |
| Manganese | 25 | Mn | 54.938 |
| Iron | 26 | Fe | 55.845 |
| Cobalt | 27 | Co | 58.933 |
| Nickel | 28 | Ni | 58.693 |
| Copper | 29 | Cu | 63.546 |
| Zinc | 30 | Zn | 65.38 |
| Gallium | 31 | Ga | 69.723 |
| Germanium | 32 | Ge | 72.63 |
| Arsenic | 33 | As | 74.922 |
| Selenium | 34 | Se | 78.971 |
| Bromine | 35 | Br | 79.904 |
| Krypton | 36 | Kr | 83.798 |
| Rubidium | 37 | Rb | 85.468 |
| Strontium | 38 | Sr | 87.62 |
| Yttrium | 39 | Y | 88.906 |
| Zirconium | 40 | Zr | 91.224 |
| Niobium | 41 | Nb | 92.906 |
| Molybdenum | 42 | Mo | 95.95 |
| Technetium | 43 | Tc | (98) |
| Ruthenium | 44 | Ru | 101.07 |
| Rhodium | 45 | Rh | 102.906 |
| Palladium | 46 | Pd | 106.42 |
| Silver | 47 | Ag | 107.868 |
| Cadmium | 48 | Cd | 112.414 |
| Indium | 49 | In | 114.818 |
| Tin | 50 | Sn | 118.710 |
| Antimony | 51 | Sb | 121.760 |
| Tellurium | 52 | Te | 127.60 |
| Iodine | 53 | I | 126.904 |
| Xenon | 54 | Xe | 131.294 |
| Cesium | 55 | Cs | 132.906 |
| Barium | 56 | Ba | 137.327 |
| Lanthanum | 57 | La | 138.906 |
| Cerium | 58 | Ce | 140.116 |
| Praseodymium | 59 | Pr | 140.907 |
| Neodymium | 60 | Nd | 144.242 |
| Promethium | 61 | Pm | (145) |
| Samarium | 62 | Sm | 150.36 |
| Europium | 63 | Eu | 151.964 |
| Gadolinium | 64 | Gd | 157.25 |
| Terbium | 65 | Tb | 158.925 |
| Dysprosium | 66 | Dy | 162.500 |
| Holmium | 67 | Ho | 164.930 |
| Erbium | 68 | Er | 167.259 |
| Thulium | 69 | Tm | 168.934 |
| Ytterbium | 70 | Yb | 173.045 |
| Lutetium | 71 | Lu | 174.967 |
| Hafnium | 72 | Hf | 178.49 |
| Tantalum | 73 | Ta | 180.948 |
| Tungsten | 74 | W | 183.84 |
| Rhenium | 75 | Re | 186.207 |
| Osmium | 76 | Os | 190.23 |
| Iridium | 77 | Ir | 192.217 |
| Platinum | 78 | Pt | 195.084 |
| Gold | 79 | Au | 196.967 |
| Mercury | 80 | Hg | 200.592 |
| Thallium | 81 | Tl | 204.383 |
| Lead | 82 | Pb | 207.2 |
| Bismuth | 83 | Bi | 208.980 |
| Polonium | 84 | Po | (209) |
| Astatine | 85 | At | (210) |
| Radon | 86 | Rn | (222) |
| Francium | 87 | Fr | (223) |
| Radium | 88 | Ra | (226) |
| Actinium | 89 | Ac | (227) |
| Thorium | 90 | Th | 232.038 |
| Protactinium | 91 | Pa | 231.036 |
| Uranium | 92 | U | 238.029 |
| Neptunium | 93 | Np | (237) |
| Plutonium | 94 | Pu | (244) |
| Americium | 95 | Am | (243) |
| Curium | 96 | Cm | (247) |
| Berkelium | 97 | Bk | (247) |
| Californium | 98 | Cf | (251) |
| Einsteinium | 99 | Es | (252) |
| Fermium | 100 | Fm | (257) |
| Mendelevium | 101 | Md | (258) |
| Nobelium | 102 | No | (259) |
| Lawrencium | 103 | Lr | (262) |
| Rutherfordium | 104 | Rf | (263) |
| Dubnium | 105 | Db | (268) |
| Seaborgium | 106 | Sg | (271) |
| Bohrium | 107 | Bh | (270) |
| Hassium | 108 | Hs | (270) |
| Meitnerium | 109 | Mt | (278) |
| Darmstadtium | 110 | Ds | (281) |
| Roentgenium | 111 | Rg | (281) |
| Copernicium | 112 | Cn | (285) |
| Nihonium | 113 | Nh | (286) |
| Flerovium | 114 | Fl | (289) |
| Moscovium | 115 | Mc | (289) |
| Livermorium | 116 | Lv | (293) |
| Tennessine | 117 | Ts | (294) |
| Oganesson | 118 | Og | (294) |
Since there is no “natural” abundance for lab-created trans-uranium elements, atomic mass for elements 93-118 listed on the periodic table is the longest-lived isotope.
Los Alamos National Laboratory
References
- https://www.britannica.com/science/atomic-weight
- https://sciencenotes.org/periodic-table-with-atomic-mass/
- https://byjus.com/chemistry/atomic-mass-of-elements/
- https://www.lenntech.com/periodic/mass/atomic-mass.htm
