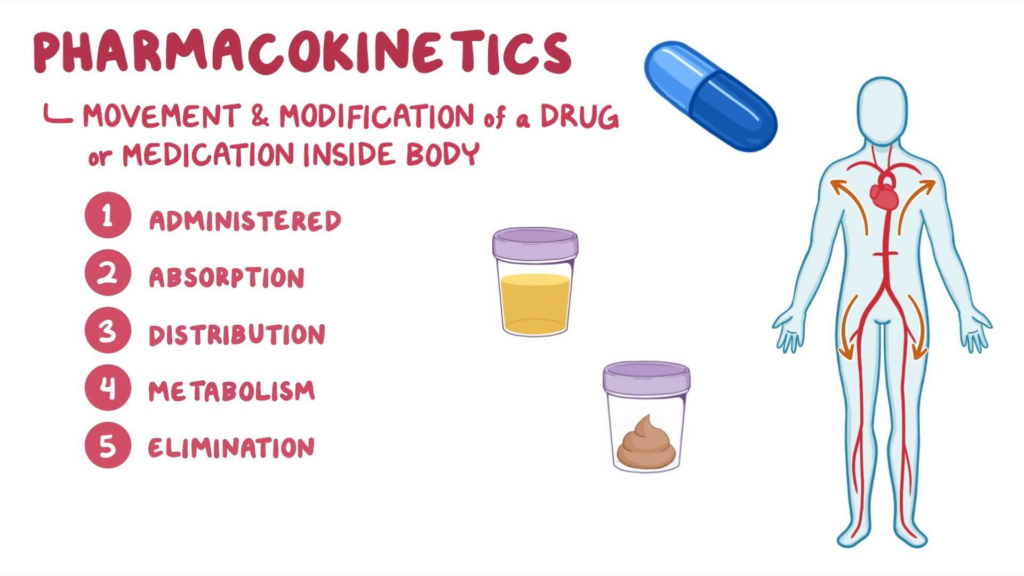
Pharmacokinetics, which is the process by which medications enter, pass through, and exit the body. Pharmacokinetics is derived from the ancient Greek terms “pharmakon” and “kinetikos,” which mean “drug” and “putting into motion,” respectively. It is one of the major fields of pharmacology that studies how the body reacts to and impacts pharmacological substances in the body.
Pharmacokinetics is essential for guaranteeing the safety of clinical medications used to treat a wide range of ailments and diseases. It investigates what happens to a drug once it enters the body, specifically how it is absorbed, distributed, metabolized, and removed. It is an important stage in the drug delivery process that ensures pharmaceuticals are both safe and effective.
Interesting Science Videos
What is Pharmacokinetics?
Pharmacokinetics is the quantitative study of how drugs move into, through, and out of the body. The pharmacokinetic qualities of the medicine determine its concentration at the site of action, which in turn affects the intensity of response.
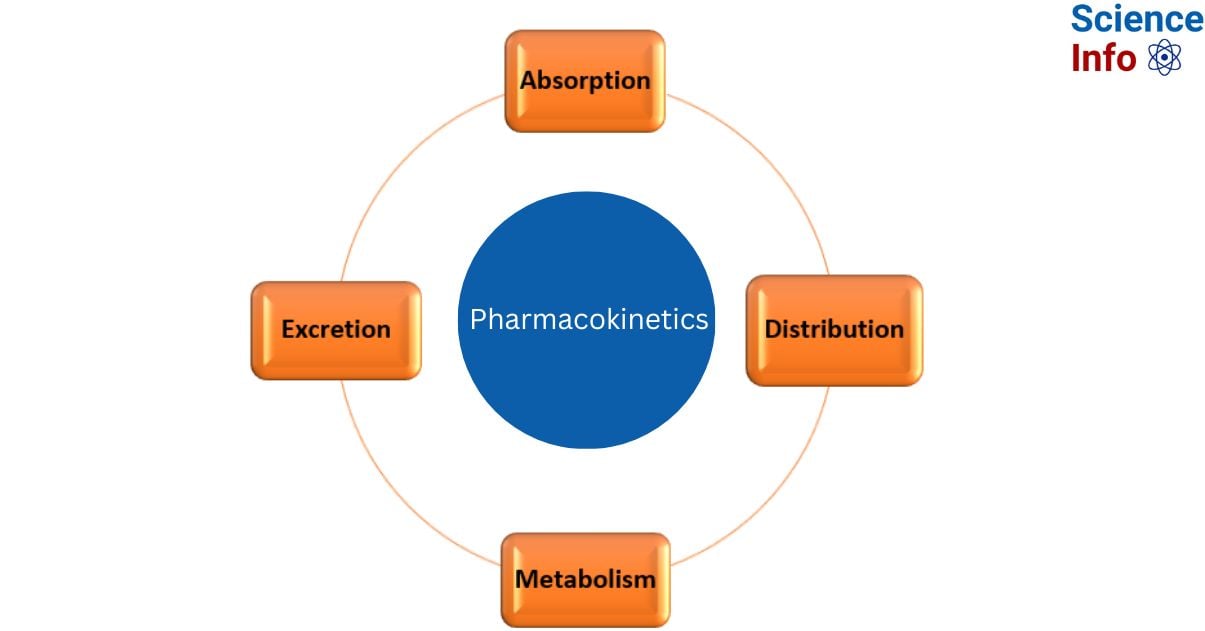
Pharmacokinetics is the study of how the body interacts with drugs over the course of their administration. In contrast to pharmacodynamics, which looks more carefully at how a medicine affects the body, this is closely connected to but clearly distinct from it. This field focuses on four major parameters: absorption, distribution, metabolism, and excretion (ADME).
The four steps include:
Absorption refers to how the drug gets from the administration site to the place of action.
Distribution refers to the how drug’s travel via the bloodstream to numerous body regions.
Metabolism refers to the process by which the drug breaks down.
Excretion refers to the process by which a medication is removed from the body.
Four Stages of Pharmacokinetics
Absorption
In pharmacology, absorption is the process by which a medication moves from its delivery point to the bloodstream. The chemical composition of a drug, as well as the environment in which it is administered, influence the rate and amount of drug absorption. There are several factors that can influence medication absorption.
Drug formulation: The small intestines absorb the vast majority of the medication. Acidic compounds are absorbed in acidic environments, whereas basic substances are better absorbed in alkaline environments.
Drug-Food Interactions: These interactions occur because the presence of food or other medications in the stomach can affect drug absorption. It sometimes increases the rate of absorption, and sometimes it combines to generate insoluble complexes that are not absorbable.
Route of administration: This can influence the rate at which the chemical is absorbed by the body.
The method of delivery (e.g., oral, intravenous, inhalation) of a medicine determines its bioavailability, which is the percentage of the active form of a drug that enters the bloodstream and successfully reaches its target location.
When a drug is administered intravenously, no absorption occurs, and bioavailability is 100% since the active form of the medicine is supplied straight to the systemic circulation. Orally given drugs, on the other hand, have inadequate absorption and hence deliver less drug to the site of action. Many orally taken medications, for example, are processed in the gut wall or liver before reaching the bloodstream. This process, known as first-pass metabolism, limits medication absorption.
Distribution
The process of drug distribution is significant because it influences how much medication ends up in active sites, and consequently drug efficacy and toxicity. A medication will migrate from the absorption site to other tissues in the body, including brain tissue, fat, and muscle. Many factors could play a role in this, including blood flow, lipophilicity, molecular size, and how the drug interacts with blood components such as plasma proteins.
This mechanism is significant because it influences how much of a drug ends up in active sites, which impacts drug efficacy and toxicity. A medication will migrate from the absorption site to tissues throughout the body, including brain tissue, fat, and muscle. Many factors may influence this, including blood flow, lipophilicity, molecular size, and how the drug interacts with blood components such as plasma proteins. Furthermore, anatomical barriers exist in specific organs, such as the blood-brain barrier, which prevents certain medications from entering brain tissue. Drugs with specific properties, such as high lipophilicity, small size, and molecular weight, will be more likely to penetrate the blood-brain barrier
Metabolism
Metabolism is the process by which the body converts a medication into its following components. This is frequently utilized to transform the drug into more water-soluble compounds that may be cleared by the kidneys; however, in the case of prodrug delivery, such as codeine, metabolism may be necessary to convert the drug into active metabolites.
Different metabolic techniques can occur in many parts of the body, including the gastrointestinal tract, skin, plasma, kidneys, and lungs, but the majority of metabolism occurs in the liver via phase I (CYP450) and phase II (UGT) reactions. Phase I reactions often convert compounds into polar metabolites by oxidation, allowing Phase II conjugation reactions to occur. Most frequently, these activities inactivate the drug, converting it into a more hydrophilic metabolite that can be eliminated in the urine or bile.
The components that influence drug metabolism include:
Genetics impact whether a person can digest medications more quickly or slowly.
Age can have an impact on liver performance; the elderly have lower liver function and may process medications more slowly, raising the risk of intolerability. Newborns and newborns have undeveloped liver function and may require special dose consideration.
Drug interactions can reduce or increase drug metabolism by enzyme inhibition or activation.
Metabolism in pharmacokinetics can be divided into two phases:[8]
Phase 1: The medication is inactivated through chemical reactions in the body such as oxidation, reduction, or hydrolysis. This causes the medicine to become totally or partially inactive.
Phase 2: It comprises chemical reactions that convert the medication into soluble molecules that can be easily eliminated. Some medications go through only one phase, however the majority go through the first phase before moving on to the second.
Excretion
The process by which a medication is eliminated from the body is called excretion. In rare situations, some medicines may never be entirely eliminated from the body. They then form an irreversible accumulation in the tissues.
The facts that influence excretion are:
Direct renal impairment can prolong the half-life of certain medicines and necessitate dose changes.
Age may influence excretion rates and drug dosing.
Pathologies affecting renal blood flow, such as congestive heart failure and liver illness, might impair medication excretion efficiency.
Why Pharmacokinetic Studies are Important?
Pharmacokinetic studies investigations enable for early evaluation of a drug‘s ADME characteristics. They also provide essential information about the effects of dietary interactions (in orally delivered pharmaceuticals), drug-drug interactions, and organ impairment on drug disposition. The foundational information obtained from pharmacokinetic studies studies helps decide whether additional investigations are required, as some types of clinical trials may not be useful or relevant for all medications. Pharmacokinetic studies studies also aid in the identification of appropriate dose for future research and real-world applications. Pharmacokinetic studies data should be acquired early and late in development to better understand a drug’s ADME features in broader research populations.
To develop the best dose regimen, identify factors that can produce inter-individual variability (variability noticed between individuals) or intra-individual variability (variability found inside the same individual). Researching a drug’s pharmacokinetic qualities early in (and throughout) development increases the likelihood of downstream success in both clinical studies and real-world patients.
Video on Pharmacokinetics
References
- https://www.news-medical.net/health/Pharmacokinetics.aspx
- https://www.certara.com/knowledge-base/what-is-pharmacokinetics/
- https://www.merckmanuals.com/professional/clinical pharmacology/pharmacodynamics/overview-of-pharmacodynamics
- https://www.aston.ac.uk/study/courses/pharmacokinetics-msc
- https://pharmaeducation.net/difference-between-pharmacokinetics-and-pharmacodynamics/
- https://www.msdmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-absorption
- https://genomind.com/providers/introduction-to-pharmacokinetics-four-steps-in-a-drugs-journey-through-the-body/
- Grogan S, Preuss CV. Pharmacokinetics. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557744/

