Phosphate fertilizer is one of the necessary components for crop growth. It is one of the most essential components influencing crop growth and life activities, with phosphorus being an important component of crop protoplasm. Phosphate fertilizers are created from phosphorites, which can be sedimentary or magmatic. Apatite is the most significant mineral.
Phosphate fertilizers are primarily generated from phosphate rock, a natural material with high phosphorus concentrations. Furthermore, the phosphate fertilizer production line may turn phosphate rock into fertilizer by milling, acidification, secondary aging, and granulation.
Interesting Science Videos
Preparation of phosphate fertilizer
Acid is added to powdered or pulverized phosphate rock to generate phosphate fertilizers. Sulfuric acid produces single or normal phosphate (SSP) with a phosphorus content of 16-21% as phosphorous pentoxide (P2O5). When phosphate rock is acidulated with phosphoric acid, triple phosphate (TSP) is formed. TSP contains 43-48% phosphorus as P2O5).
Single super phosphate (SSP)
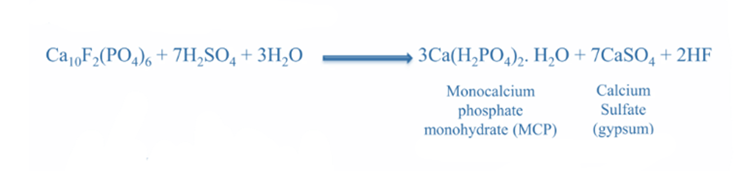
SSP is created by reacting non-soluble fluorapatite (a mineral found in phosphate rock) with sulfuric acid to produce soluble monocalcium phosphate and calcium sulfate. For more than a century, it was the primary fertilizer, supplying more than 60% of the world’s phosphate.
During its preparation, the grade of phosphate rock determines the product’s quality. Because of the rock’s limited reactivity, fine grinding is required. Phosphate rock is finely pulverized to less than 100 meshes in size. The advantages of grinding phosphate rock to fine powder are as follows:
- Increase the reaction rate
- Less sulfuric acid is required.
- A higher quality of product is obtained in better condition.
Reaction involved
Benefits of SSP
1. The method is straightforward, requiring little technical expertise and a small capital expenditure.
2. SSP provides two secondary elements, Sulphur and Calcium, that are occasionally lacking in soil.
Triple superphosphate (TSP)
TSP was one of the first high-analysis phosphorus (P) fertilizers to become widely employed in the twentieth century. It is also known as calcium dihydrogen phosphate and monocalcium phosphate, [Ca(H2PO4)2.H2O]. Despite its strong track record as a P source, its use has decreased as other P fertilizers have gained popularity.
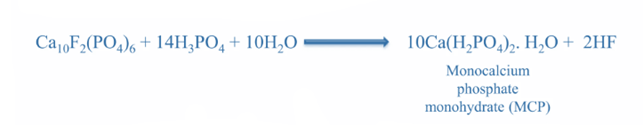
It has the greatest P content among non-nitrogen (N) dry fertilizers. Because more than 90% of the total P in TSP is water soluble, it is readily available for plant absorption. The concentrated soil solution becomes acidic as soil moisture dissolves the granule. TSP also includes 15% calcium (Ca), which adds another plant nutrient. It has almost 2.5 times the P2O5 content of SSP. The procedure is similar to that of SSP, except that phosphoric acid is used instead of sulfuric acid.
Advantages of TSP
(i) the highest analysis of any straight P product
(ii) a relatively simple production process,
(iii) some of the P2O5 used in the process is from PR,
(iv) less byproduct gypsum per tonne of P2O5 formed, and
v) TSP economics favor larger, more efficient plants near the source of PR.
Disadvantages of TSP include
TSP has the following disadvantages
(i) It takes a long time and space to cure.
(ii) it is acidic, which reduces bagged product storability
(iii) it is incompatible with other products such as urea.
(v) requires very finely ground PR for complete reaction.
(vi) production efficiencies and energy requirements favor ammonium phosphates.
Ammonium Phosphate
Ammonium phosphates are a kind of fertilizer materials made from phosphoric acid and anhydrous ammonia.
Ammonium phosphates derived from wet-process phosphoric acid have excellent physical, storage, and handling properties. A small quantity of contaminants in wet-process acid is advantageous. Granulation and storage properties are superior to those of ammonium phosphates produced on electric-furnace acid. Furthermore, ammonium phosphates improve production efficiency, energy efficiency, and a high degree of conversion to soluble phosphates. The major reason they are appealing to phosphate producers is that they are reasonably simple to create.
Monoammonium Phosphate
Monoammonium phosphate (MAP) is formed when one mole of ammonia reacts with one mole of phosphoric acid. The basic process for manufacturing MAP is also simple, involving the ammoniation of wet-process phosphoric acid to a 1:1 mole ratio of NH3:H3PO4.
NH3 + H3PO4 → (NH4)H2PO4
Nongranular MAP is typically utilized as an ingredient in the manufacture of granular NPK mixes or other nongranular products/processes.
Advantages of Monoammonium Phosphate
MAP provides numerous advantages, including the following:
(i) There is no single commodity grade for MAP.
(ii) lower quality, higher impurity acid can be utilized in production
(iii) It contains more P2O5 than other solid phosphate products, lowering storage and shipping costs.
(iv) Less risk of ammonia loss during manufacturing
(v) Less ammonia is transported to the production site than in DAP production.
Diammonium phosphate
Diammonium phosphate is formed when two moles of ammonia are mixed with one mole of phosphoric acid. The basic procedure for generating DAP is relatively simple. Phosphoric acid is neutralized by approximately two moles of ammonia.
H3PO4 + 2NH3 → (NH4)2HPO4
Advantages of Diammonium phosphate
DAP is the most frequently used phosphorus fertilizer on earth. It is popular because of its high nutritious content and outstanding physical characteristics. DAP is an effective plant nutrition source of phosphate (P) and nitrogen (N). It supplies the proper phosphorus and nitrogen ratio for grain production such as wheat, barley, fruits, and vegetables.
References
- https://www.weekand.com/home-garden/article/phosphate-fertilizer-types-18053367.php.
- https://www.indorama.com/products/phosphate-fertilizers.
- https://www.ifc.org/content/dam/ifc/doc/1990/phosfer-ppah.pdf.
- https://www.slideshare.net/AdeelHasnain1/phosphate-based-fertilizers.
- https://www.slideshare.net/VISHALTHAKRE3/superphosphate-79176464.
