Group 17 of the periodic table is named Halogens as they all produce sodium salts with similar properties. In Greek, halo means salt, and genes mean generating, so salt-producing is a collective meaning of the term.
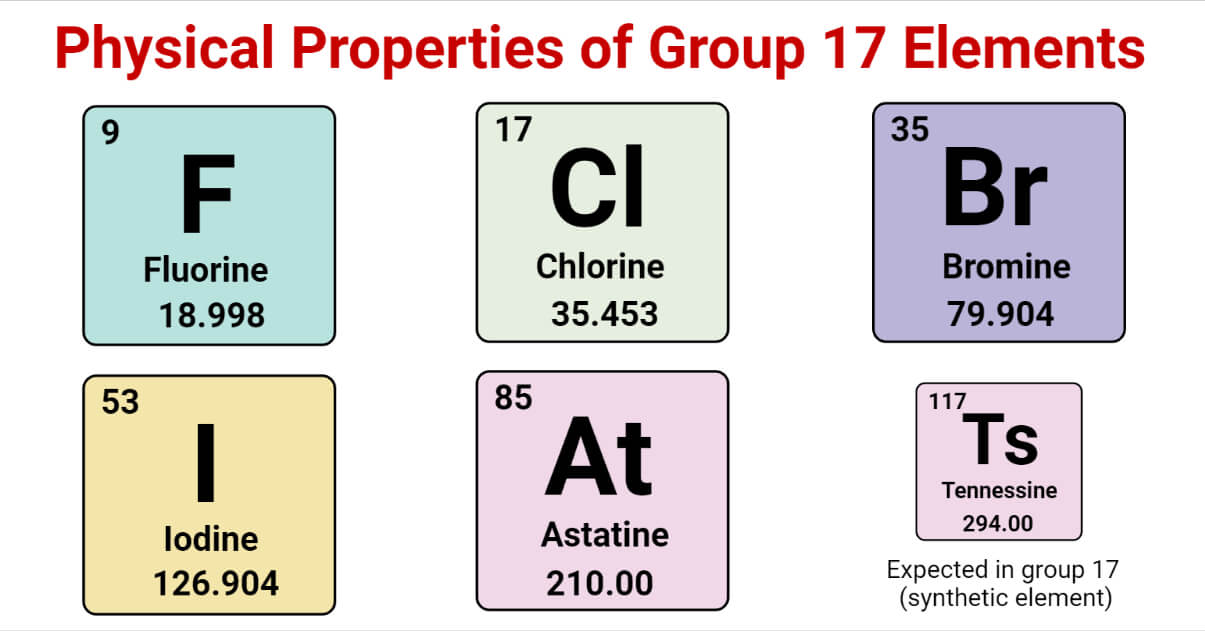
Halogens are also known as Group 7 or Group 17.
On the periodic table, the halogens are to the left of the noble gases.
Group 17 of the periodic table consists of fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Tennessine is extremely unstable; emphatically, it exists only for fractions of a second at a time. In addition, this is combined with its high cost, which means that many of its properties have yet to be discovered. Specifically, they are only speculative.
Similarly, astatine is unstable, with a maximum half-life of slightly more than eight hours. Many of the properties of astatine have also not been observed. Despite being radioactive and having only short-lived isotopes, astatine behaves similarly to iodine and is commonly classified as a halogen.
In fact, no pure sample of astatine has ever been collected because any specimen would vaporize due to the heat generated by its own radioactivity.
Interesting Science Videos
Occurrence of Halogens
Because halogen elements have seven valence electrons, a complete octet requires only one more electron. As a result, they are more reactive than other nonmetallic groups.
Except for astatine, all are abundant in the earth’s crust as halide ions, X.
Fluorine and chlorine are relatively abundant, whereas bromine and iodine are relatively scarce.
Fluorine is the thirteenth most abundant element in earth’s crystal rocks.
Chiefly, fluorines are mostly found as insoluble fluorides. However, at room temperature, it exists in the gaseous state.
Although it is found as insoluble fluorides, cryolites, fluorspar, and fluorapatite. Fluorine can also be found in soil, plants, stream water, and animal bones and teeth.
Chlorine is the 20th most abundant element in the Earth’s crust by weight.
At room temperature, it is in a gaseous state. The ocean’s water contains 1.5 percent sodium chloride by weight.
Sodium chloride can be found in the ocean’s dry bed, whereas, at room temperature, iodine is a solid.
Chiefly, chlorides, bromides, and iodides of chlorine, bromine, and iodine are found in ocean water. Also, at room temperature, bromine exists as a liquid.
Physical Properties of Elements of Group 17
Electronic Configuration of Elements of Group 17
Firstly, the electronic configuration is simply the arrangement of an atom’s electrons in its orbitals. The study of an atom’s or molecule’s electronic configuration significantly aids in understanding trends in an element’s physical and chemical properties. Accordingly, the number of electrons in an element’s outermost shell can be used to calculate its reactivity and the types of bonds it forms.
The electronic configurations of the elements of group 17’s outermost shells are ns2 and np5. As a result, the outermost shell contains 7 electrons. Eventually, the outermost shell is one electron short of achieving an octet. Hence, these elements require one more electron to achieve an octet or ideal gas configuration.
Group 17 elements have the general electronic configuration ns2np5.
The halogen group electronic configuration is as follows:
| Element | Symbol | Atomic Number | Electronic Configuration |
| Fluorine | F | 9 | [He]2s22p5 |
| Chlorine | Cl | 17 | [Ne]3s23p5 |
| Bromine | Br | 35 | [Ar]3d104s24p5 |
| Iodine | I | 53 | [Kr]4d105s25p5 |
| Astatine | At | 85 | [Xe]4f145d106s26p5 |
| Tennessine | Ts | 117 | [Rn]5f146d107s27p5 (predicted) |
Atomic and Ionic Radii of Elements of Group 17
Atomic radii are the distances between the center of an atom and the outermost shell containing electrons whereas ionic radii are the dimensions of an atom’s ion.
Altogether, the atomic and ionic radii increase as the atomic number increases. This is because the number of electron shells has increased. Because of their maximum effective nuclear charge, halogens have the smallest atomic radii in their respective periods.
| Elements | F | Cl | Br | I | At |
| Covalent Radius (pm) / (angstroms) | 71 / 0.57 | 99 / 1.02 | 114 / 1.2 | 133 / 1.39 | 150 / 1.5 |
| Ionic Radius (pm) / (angstroms) | 133 / 1.19 | 181 / 1.67 | 196 / 1.82 | 220 / 2.06 | – |
The radius of a halide ion is always greater than that of a halogen atom. This is because the halide ion is formed by the atom gaining one electron. As a result, the number of electrons increases while the nuclear charge remains unchanged. As a result, the same nuclear charge acts on a greater number of electrons than exists in the neutral atom. The effective nuclear charge per electron is reduced, and thus the nucleus holds the electron cloud less tightly.
Ionization Energies of Elements of Group 17
Ionization energy is the amount of energy required to remove an electron from its valence shell.
Halogens have extremely high ionization energies thus this indicates that they have a low proclivity to lose electrons.
The ionization enthalpy significantly decreases as one moves down the group from fluorine to astatine. This is because of the gradual increase in atomic size, which is greatest for iodine.
Fluorine has a higher ionization energy than any other halogen because of its small size, which increases the attraction between the core and the valence shell.
| Element | F | Cl | Br | I | At |
| First Ionization Energy (KJ/mol) | 1681 | 1251.20 | 1139.90 | 1008.40 | 920 |
If they are not close to the nucleus then It takes less energy to remove outer valence electrons. As a result, because there are more energy levels, the energy required to remove an outermost electron is lower for elements in the lower part of the group. Furthermore, the high ionization energy renders the element non-metallic. Because iodine and astatine have metallic properties, the ionization energy decreases as one moves down the group.
The trend in ionization energy of elements is group 17: (At < B < Br < Cl < F)
Melting and Boiling Points of Elements of Group 17
The melting and boiling points of halogens increase with increasing atomic number accordingly as we move down the group. Because the size of the atoms increases as we move down the group, so does the Van Der Waals force of attraction.
| Element | F | Cl | Br | I | At |
| Boiling Point (°C) | -188 | -35 | 58.8 | 184 | 337 |
| Melting Point (°C) | -220 | -101 | -7.2 | 114 | 302 |
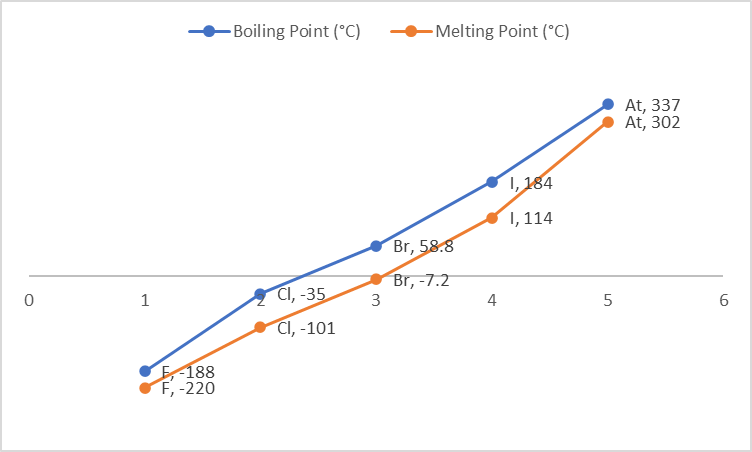
Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine and astatine are solids.
As molecules grow in size, there are obviously more electrons that can move around and form the temporary dipoles that cause these attractions.
As molecules grow in size, their intermolecular attractions become stronger, requiring more heat energy to convert them to a liquid or a gas – and thus their melting and boiling points rise.
Electronegativity
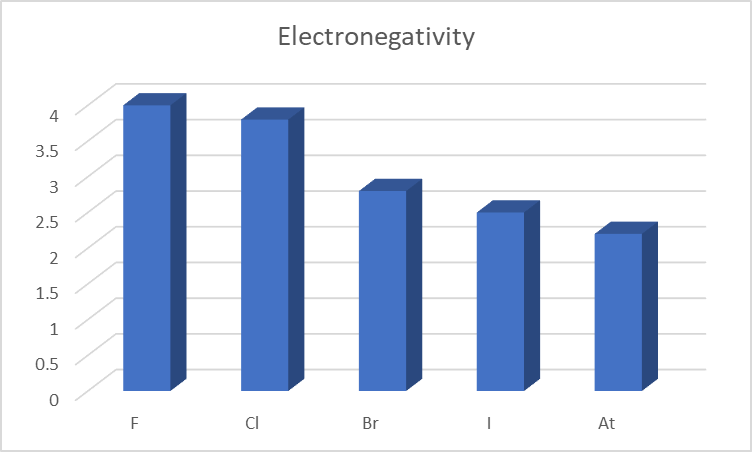
Electronegativity is the ability of an atom to attract an electron or a bonding pair of electrons. Comparatively, halogens have an extremely high electronegativity.
Due to the increase in energy levels at progressively lower levels, the number of valence electrons in an atom increases down the group. Because the electrons move away from the nucleus, the nucleus and electrons are less attracted to each other. Moreover, there is an increase in shielding.
Color of Elements in Group 17
The excitation between the highest occupied π* Molecular Orbital and the lowest unoccupied σ* Molecular Orbital is particularly responsible for the color of halogens.
Also, the amount of energy required for excitation is proportional to the atom’s size.
Also the energy difference between HOMO and LUMO decreases as F2 > Cl2 > Br2 > I2.
| Element | F | Cl | Br | I | At |
| Color | Light-greenish yellow | Greenish-yellow | Brown red | Dark violet | – |
As a result, it requires high excitation energy and absorbs violet light (high energy), giving it a light greenish-yellow appearance. On the other hand, Iodine requires significantly less excitation energy and absorbs low-energy yellow light. Therefore, appears dark violet.
Oxidation States of Halogens
All elements in group 17 have seven electrons in their valence shell. Hence, to complete their octet, these elements require one electron. As a result, they can obtain the noble gas configuration by either gaining one electron to form the uni-negative ion, X, or by sharing electrons with other atoms. Therefore, they exhibit an oxidation state of -1 or + 1.
| Element | F | Cl | Br | I | At |
| Oxidation States | -1 | -1, +1, +3, +5, +7 | -1, +1, +3, +5, +4 | -1, +1, +5, +7 | -1,+1, +3, +5,+7 |
Except for fluorine, the valence shells of bromine, chlorine, and iodine contain free d-orbital. As a result, they have different oxidation states such as +1, +3, +5, +7, and -1.
Shortly, oxoacids, interhalogens, and oxides are examples of positive oxidation states.
Electron Gain Enthalpies of Group 17 Elements
Electron gain enthalpy is the energy released when an electron is added to an isolated gaseous atom. The electron gain enthalpy of halogens is negative. However, as one moves down the group, the electron gain enthalpy becomes less negative.
In their respective periods, all of these have the highest negative electron gain enthalpies. This is because the atoms of these elements have one less electron than the stable noble gas (ns2np6) configurations. As a result, they have the greatest inclination to accept an additional electron.
In a group, electron gain enthalpy decreases from top to bottom.
The effect of increasing atomic size is much greater than the effect of increasing nuclear charge, so the additional electron feels less attraction from the large atom. As a result, the electron gain enthalpy decreases.
| Elements | F | Cl | Br | I |
| Electron Gain Enthalpy (KJ/mol) | -333 | -348 | -324 | -295 |
In brief, factors on which electron gain enthalpy depends are:
- Atomic Size
- Nuclear charge
- Electronic configuration
Metallic and Non-metallic character of Halogens
All halogens have a non-metallic character due to their extremely high ionization energy values.
Markedly, as we move down the group, the non-metallic character decreases.
Density of Group 17 Elements
As the molecular weight increases down the group, density decreases.
| Elements | F | Cl | Br | I |
| Density (g/cm3) | 0.0017 | 0.0032 | 3.1028 | 4.9333 |
Also, for the summary of the properties of elements of group 17 have a look at the video.
References
- Smith, D. (1990). Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry (Cambridge Texts in Chemistry and Biochemistry). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511622922
- Lee, J D. Concise Inorganic Chemistry. London: Blackwell Science, 2006. Print.
- Cotton, F A, and F A. Cotton. Advanced Inorganic Chemistry. , 1999. Print.
- Mingos, D. M. P. Essential Trends in Inorganic Chemistry. Oxford University Press, 1998.
- https://byjus.com/jee/alkaline-earth-metals/
- https://www.britannica.com/science/alkaline-earth-metal/Physical-and-chemical-behaviour
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_sBlock_Elements/Group__2_Elements%3A_The_Alkaline_Earth_Metals/1Group_2%3A_Chemical_Reactions_of_Alkali_Earth_Metals/Group_2%3A_General_Properties
- https://www.vedantu.com/chemistry/group-17-elements
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/2-inorganic-chemistry/2-3-group-17/2-3-1-physical-properties-of-the-group-17-elements/

