The pKa value of a molecule describes its acidity. A pKa table simplifies pKa values. It determines an acid’s strength by how firmly a proton is retained by a Bronsted acid. The lower the pKa value, the more powerful the acid and its capacity to transfer protons. define a molecule’s acidity.
Interesting Science Videos
What is pKa?
pKa is the negative base-10 logarithm of a solution’s acid dissociation constant (Ka).
Both pKa and Ka provide information in certain ways, however the Ka value is a tiny decimal number that is easy to deal with, whereas Ka contains exponents and employs scientific notation.
Relationship Between pKa and Ka
- pKa = -log10Ka
- Ka = 10-pKa
How to use a pKa Table
- pKa values have been recorded for a vast variety of compounds and are mainly connected to the molecule’s most acidic functional group. A pKa table simplifies pKa values.
- A low pKa value (for example, -10) implies a strong acid. A high pKa value (for example, 50) implies a weak acid.
- A conjugate acid and a conjugate base are formed when an acid and a base swap a bond to H.
- Acid-base reactions are always represented as equilibria, which occur in either a forward or backward direction. A stronger acid and a stronger base generate a weaker acid and a weaker base, which is the preferred direction for an acid-base interaction.
- By comparing the pKa of the acid and the conjugate acid, one may determine which way is preferred. The formation of the weaker acid (molecule with higher pKa) will be preferred.
- These principles can also be applied to other reactions (for example, substitution reactions).
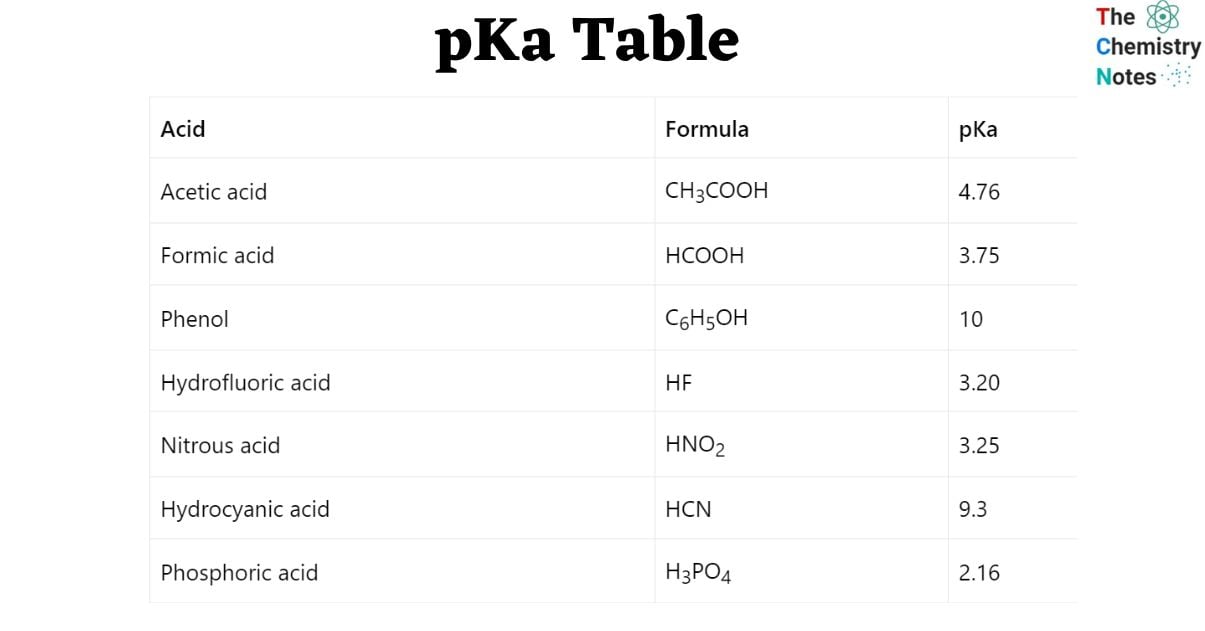
pKa Table for common acids
| Acid | Formula | pKa |
| Acetic acid | CH3COOH | 4.76 |
| Formic acid | HCOOH | 3.75 |
| Phenol | C6H5OH | 10 |
| Hydrofluoric acid | HF | 3.20 |
| Nitrous acid | HNO2 | 3.25 |
| Hydrocyanic acid | HCN | 9.3 |
| Phosphoric acid | H3PO4 | 2.16 |
| Alkane | CH4 | 50 |
| Alkyne | CH☰CH | 25 |
| Oxalic acid | H2C2O4 | 1.2 |
| Trifluoro acetic acid | CF3COOH | 0.52 |
| Nitric acid | HNO3 | -1.4 |
| Water | H2O | 14 |
| Sulfuric acid | H2SO4 | -3 |
| Hydrogen sulphide | H2S | 7.05 |
| Hydrochloric acid | HCl | -7 |
| Carbonic acid | H2CO3 | 6.35 |
| Perchloric acid | HClO4 | -10 |
| Hydroiodic acid | HI | -9.5 |
| Hydronium ion | H3O+ | -1.74 |
| Amine | NH3 | 35 |
| Boric acid | H3BO3 | 9.15 |
| Cyanic acid | HOCN | 3.46 |
pKa Table Organic Chemistry
Most organic acids have pKa values between 5-60. While it is difficult to determine the pKa of every organic molecule, understanding the pKa (and acidity) based on the functional groups involved is extremely important because the same functional groups typically have comparable pKa.
Table here shows the approximate ranges of pKa values for seven key functional groups, which may be used to predict and understand the acidity of any organic molecule.
| Functional Group | pKa |
| Carboxylic acid | ∼5 |
| Alcohol | ∼16 |
| Aldehyde/ Ketone | ∼16 -20 |
| Alkyne | ∼25 |
| Amine | ∼35-40 |
| Alkene | ∼45 |
| Alkane | ∼>50 |
How to Predict the Position of Equilibrium in Organic Acid-Base Reactions Using values in pKa table
To estimate the location of equilibrium in the following reaction, we must first decide what will be the acid and what will be the base on both the left and right. The conjugate base is formed when the base accepts the acidic proton. This means that the hydroxide ion serves as a base and acetic acid serves as an acid. In the opposite reaction, however, water acts as an acid and acetic ion acts as a base. The acetic ion is an acetic acid conjugate base, while the hydroxide is a water conjugate base.
When we compare the pKa values of these acids (acetic acid and water), we observe that the left side is 4.75 and the right side is 15.7. Because the equilibrium favors the weaker acid, the acid with the higher pKa value will win. This will be water with a higher pKa value in this situation. This signifies that the equilibrium is to the right.
References
- https://chemistryscore.com/use-pka-table/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_I_(Cortes)/11%3A_Bronsted_Acid-Base_Chemistry/11.04%3A_pKa_Table
- https://byjus.com/chemistry/pka/#:~:text=FAQs-,Definition%20of%20pKa,ability%20to%20donate%20its%20protons.
- Atkins, Peter; de Paula, Julio (2006). Physical Chemistry. Oxford. ISBN 978-0198700722.
- Denbigh, K. (1981). “Chapter 4.” The Principles of Chemical Equilibrium (4th ed.). Cambridge: Cambridge University Press. ISBN 978-0-521-28150-8.
- https://sciencenotes.org/what-is-pka-in-chemistry-acid-dissociation-constant/
- https://www.masterorganicchemistry.com/2010/09/29/how-to-use-a-pka-table/#five
- https://organicchemistrydata.org/hansreich/resources/pka/
