Polyethylene is a polymeric molecule often known as plastic, which is widely used in packaging. It is made up of a chain of ethylene molecules that have been linked together by a polymerization event. It is sometimes called polythene or polyethylene. Polyethylene is becoming an essential component of our daily lives. In 1898, German scientist Hans von Pechmann presented it for the first time. Polyethylene is regarded as one of the most common environmental contaminants nowadays due to its inability to degrade.
Interesting Science Videos
What is polyethylene?
- Commercial plastic that is most commonly used is polyethylene (PE).
- Its molecular chain configuration determines how many various forms it is available in.
- Because polyethylene is composed of many units of the same type of molecule—ethylene—it is categorized as a homopolymer.
- Tough and resistant to abrasion, polyethylene is a plastic that is easily manufactured through the use of injection and blow molding techniques. Plastic bags, water tanks, and bottles are the usual uses for it.
- The term “polyethylene” describes a class of thermoplastic homopolymers composed of molecular chains of numerous repeating ethene monomers (IUPC designation “ethene”). Plastic bags, food and drink containers, and medical uses such as knee joints are commonly made of polyethylene.
- The chemical structure of polyethylene is composed of repeating monomers formed of hydrogen and carbon atoms.
- The general formula for polyethylene is represented by (C2H4)n. Heat can be used to remold the majority of polyethylene varieties, which are thermoplastic. Certain thermoset qualities are present in certain modified polyethylene polymers, though. Cross-linked polyethylene, often known as PEX, is an illustration of this type of polyethylene.
- Polyethylene comes in four basic varieties: Linear Low-Density Polyethylene (LLDPE), High-Density Polythene (HDPE), Low-Density Polyethylene (LDPE), and Ultra-High Molecular Weight Polyethylene (UHMWPE).
- Polyethylene polymer’s linear and simple structure gives it various qualities such as flexibility, chemical resistance, and electrical insulating capabilities. Polyethylene is used in a variety of applications, including packaging, containers, pipes, and medical equipment, and comes in two variations: Low-Density Polyethylene (LDPE) and High-Density Polyethylene (HDPE).
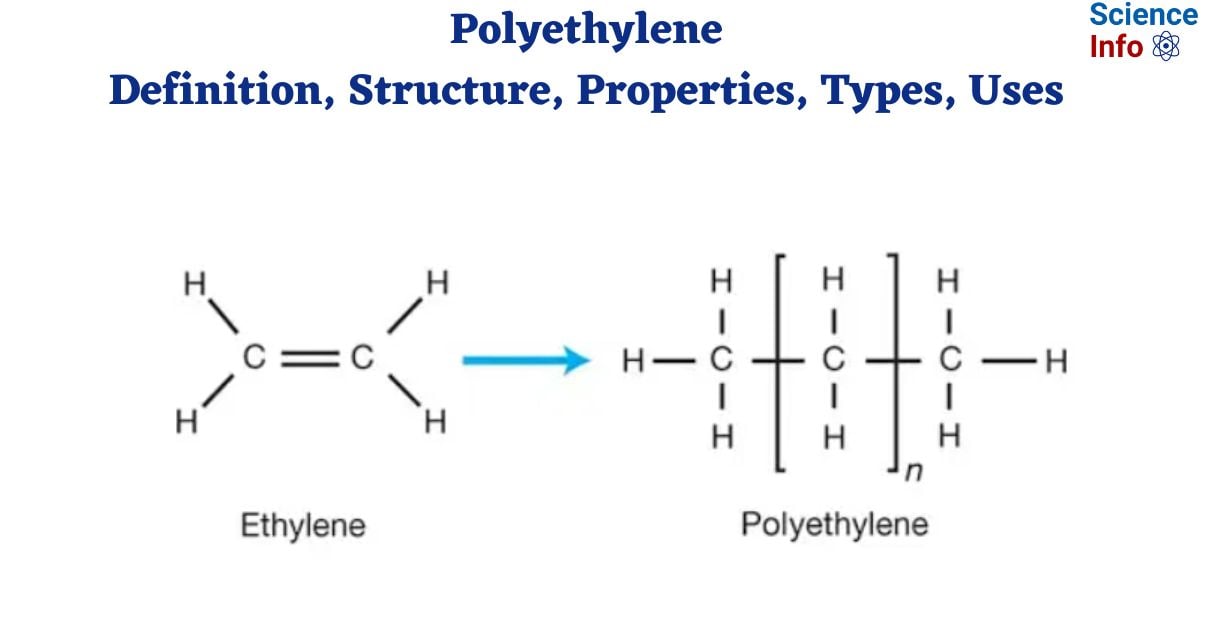
Structure of polyethylene
The repeating unit that is formed from the monomer ethylene forms the basis of the molecular structure of polyethylene. A double bond forms between two carbon atoms and two hydrogen atoms are connected to each carbon in ethylene (C2H4), a simple hydrocarbon. Ethylene polymerizes to create a lengthy chain of repeating units.
The chemical Formula for Polyethylene is (C2H4)n, where “n” represents the number of repeating units in the polymer chain.
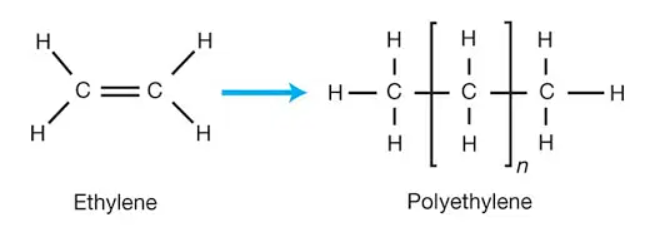
Preparation of polyethylene
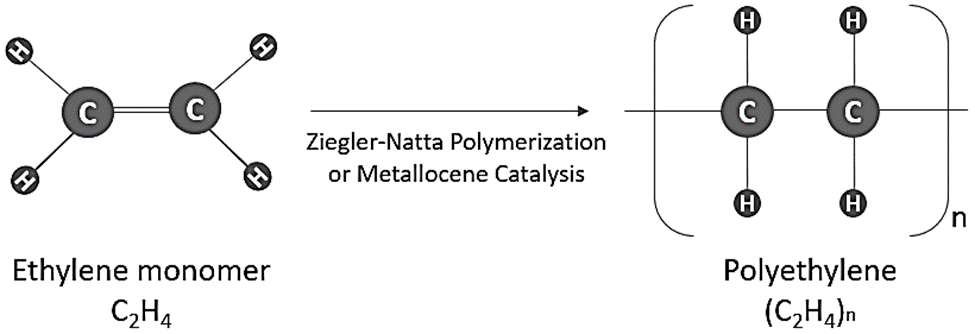
Ethylene, an organic hydrocarbon with the chemical formula C2H4 and the IUPAC name ethene, is the main component of polyethylene. Less than 5 parts per million of oxygen, water, and other alkenes are typically required for the manufacture of polyethylene. On the other hand, other substances may exist as impurities during the polymerization process. It is generally acknowledged that ethane, methane, and nitrogen can be pollutants in the process of producing polythene.
Because ethene is a highly stable chemical, appropriate catalysts are needed for its polymerization. It is significant to remember that ethylene undergoes a very exothermic transformation to become polyethylene. Ziegler-Natta catalyst, also known as titanium(III) chloride, is one of the most widely used catalysts for the polymerization of ethylene.
Properties of polyethylene
Physical properties of polyethylene
- Density: Polyethylene is characterized by its relatively low density, typically ranging from 0.91 to 0.96 g/cm³. This low density makes it lightweight, which is advantageous for applications where weight is a concern, such as packaging materials and lightweight structures.
- Flexibility: Because of its remarkable flexibility, polyethylene is easily molded, sculpted, and formed into a variety of items. Because of this characteristic, it can be used in applications including flexible packaging, plastic bags, and tubing that call for elasticity and flexibility.
- Strength: Polyethylene is surprisingly strong and durable for its elasticity. Because of its strength, it can be used in applications where durability and impact resistance are necessary, such as pipelines, containers, and automotive parts.
- Transparency: Depending on its molecular structure and processing methods, polyethylene can range from transparent to translucent or opaque. Transparent polyethylene variants are often used in applications such as clear plastic bags, shrink wrap, and bottles.
- Electrical insulation: Because of its superior electrical insulating qualities, polyethylene is frequently employed in electronic and electrical applications. It is appropriate for insulating cables, wires, and electrical components due to its high dielectric strength and low electrical conductivity.
- Chemical resistance: Polyethylene is resistant to many chemicals, including acids, bases, and organic solvents. This chemical resistance makes it suitable for applications where exposure to harsh chemicals is expected, such as chemical storage tanks, piping systems, and laboratory equipment.
- Thermal properties: Polyethylene can tolerate a broad range of temperatures without degrading significantly and demonstrates high thermal insulation qualities. Because of its thermal stability, it can be used in temperature-sensitive applications such as automobile components, thermal packaging, and insulating materials.
- Water resistance: Because polyethylene is hydrophobic, it has a high degree of resistance to water absorption. Because of this characteristic, it’s perfect for uses where water resistance is needed, like water pipes, marine buildings, and outdoor furniture.
Chemical properties of polyethylene
- Inertness: Since polyethylene is chemically inert, it doesn’t normally react with most other substances. Its stability and resilience in a variety of conditions are facilitated by its inertness.
- Polymer structure: Polyethylene comprises repeating ethylene monomer units linked together through covalent bonds, forming long chains or branches. The polymer structure can vary, resulting in different types of polyethylene with varying properties, such as high-density polyethylene (HDPE) and low-density polyethylene (LDPE).
- Hydrophobicity: Because of its non-polar molecular structure, polyethylene is extremely hydrophobic, rejecting water and other polar liquids. Its hydrophobic properties add to its water resistance and suitability for applications that are exposed to dampness and the outdoors.
- Melting point: The melting point of polyethylene varies depending on its molecular weight, crystallinity, and type. High-density polyethylene (HDPE) typically has a higher melting point (around 120°C to 130°C) compared to low-density polyethylene (LDPE), which melts at lower temperatures (around 105°C to 115°C).
- Polymerization: Usually, ethylene gas is polymerized with catalysts in a regulated environment to create polyethylene. In the polymerization process, catalysts like metallocene or Ziegler-Natta catalysts are frequently used.
- Crystallinity: Polyethylene can exhibit varying degrees of crystallinity, depending on factors such as molecular weight, branching, and processing conditions. Crystalline regions contribute to its mechanical strength and thermal properties, while amorphous regions enhance flexibility and transparency.
- High molecular weight nonpolar saturated hydrocarbons make up polyethylene. This is thought to be the cause of polyethylene’s chemical characteristics’ striking resemblance to paraffin. Notably, covalent bonds do not bind the distinct polyethylene macromolecules together. However, because of their relatively symmetric molecular architectures, these molecules crystallize. Polythene can therefore be thought of as a partly crystalline plastic. A polymer’s density and chemical stability increases with its crystallinity.
- The majority of polyethylene varieties, including LDPE, MDPE, and HDPE, have extremely good chemical resistance to acids and alkalis. Additionally resistant to weak reducing and oxidizing agents are these polymers. At higher temperatures, the majority of polyethylenes are known to dissolve in aromatic hydrocarbons like toluene or xylene.
Types of Polyethylene
- Ultra-High Molecular Weight Polyethylene (UHMWPE): The molecular chain of UHMWPE is linear and lacks side branches. Because of its exceptional toughness and abrasion resistance, it is a good fit for industrial applications. The term “ultra-high molecular weight” refers to UHMWPE because its continuous molecular chains are substantially longer than those of other polyethylenes.
- High-Density Polythene (HDPE): The molecular chain of HDPE material is linear, with little to no branching off of the main chain. In comparison to LDPE, this enables the polymer chain to fold into a dense structure, resulting in improved packing efficiency and increased crystallinity. Rigid and possessing good mechanical qualities, HDPE is a material. It appears to be opaque.
- Low-Density Polyethylene (LDPE): The linear molecular structure of HDPE is absent from LDPE. As an alternative, the primary carbon backbone may include supplementary branches that resemble the structure of the fundamental polyethylene molecule. In contrast to the linear structure of HDPE, these branches prevent the molecular chain from folding into a tightly packed structure, decreasing its packing efficiency. Softer than HDPE is LDPE. It also has a low crystallinity and a tendency to be translucent.
- Linear Low-Density Polyethylene (LLDPE): LDPE and LLDPE share a similar molecular structure. Its branches are noticeably shorter than those of LDPE, nevertheless. This indicates that the molecular chains are less prone to entangling. LLDPE exhibits very high elongation and good tensile strength. It is frequently utilized in stretch films as a result.
Uses of polyethylene
- Packaging: Because polyethylene is strong, flexible, and resistant to chemicals and moisture, it is frequently used to make packaging materials including plastic bags, shrink wrap, and containers.
- Plastic bottles: Because polyethylene is lightweight and easily molded into a variety of shapes, it is used to make a large number of beverage bottles, including water bottles.
- Insulation: Polyethylene foam is used as insulation in buildings and homes to help regulate temperature and reduce energy costs. It provides excellent thermal insulation and is resistant to moisture, making it ideal for this purpose.
- Plumbing and irrigation systems frequently use polyethylene pipes because of its flexibility, resistance to corrosion, and affordability when compared to metal pipes. They are also utilized in sewage and gas distribution systems.
- Medical Devices: Polyethylene is used in the manufacturing of various medical devices and equipment, including prosthetic limbs, joint replacements, and medical implants due to its biocompatibility and ability to be sterilized.
- Electrical Insulation: To guard against electrical conductivity and stop short circuits, thin polyethylene sheets are employed as electrical insulation in wires and cables.
- Toys and Sporting Goods: Polyethylene is used to manufacture a wide range of toys and sporting goods such as plastic balls, Frisbees, and playground equipment due to its durability and safety.
- Films: In agriculture, polyethylene films are used for silage protection, mulching, and greenhouse covers. Additionally, they are utilized in the creation of industrial wrapping materials and food packaging films.
- Automobiles: Because polyethylene is lightweight, impact-resistant, and weather-resistant, it is used in vehicle applications such as fuel tanks, bumpers, and interior trim components.
- Polyethylene sheets are used as vapor barriers and moisture barriers in construction projects to prevent water infiltration and damage to building materials.
Advantages of polyethylene
- Chemical Resistance: Because polyethylene is so resistant to chemicals, it can be used to store and move a variety of materials without deteriorating.
- Low Cost: Compared to other plastics, polyethylene is comparatively inexpensive to produce, which makes it a cost-effective choice for a range of applications.
- Durability: Polyethylene is known for its durability and toughness, making it capable of withstanding harsh environmental conditions and heavy loads without breaking or deforming easily.
- Flexibility: Polyethylene is easily molded and sculpted into a variety of shapes, which makes it useful for a wide range of goods and sectors.
- Lightweight: Because polyethylene is lightweight, it is perfect for uses where weight is an issue, including in packaging and auto parts.
- Insulation Properties: Polyethylene has excellent insulation properties, both thermal and electrical, making it suitable for use in insulation materials for buildings, electrical cables, and appliances.
- Recyclability: Polyethylene may be recycled into new items, minimizing waste and its impact on the environment. Many products made of polyethylene are also recyclable.
- Water Resistance: Polyethylene is resistant to moisture and water absorption, making it suitable for outdoor applications and storage of liquids.
Disadvantages of polyethylene
- Low-Temperature Resilience: Polyethylene is brittle and prone to shattering in cold conditions because of its weak resilience to low temperatures.
- UV Degradation: UV radiation from sunlight can cause polyethylene to degrade, which over time results in a loss of strength and color fading.
- Flammability: Polyethylene is highly flammable and can melt or burn when exposed to high temperatures, releasing toxic fumes and gases.
- Poor Adhesion: Polyethylene has a low surface energy, which makes it challenging to use coatings or adhesives to attach with other materials.
- Creep: Polyethylene can experience creep, which is the gradual deformation or sagging of the material under constant stress over time, affecting its dimensional stability in load-bearing applications.
- Paint-Difficult: Polyethylene is difficult to paint or coat because of its low surface energy, which calls for a specific surface treatment to ensure adherence.
- Environmental Concerns: Despite being recyclable, polyethylene is still a material derived from petroleum, and both its manufacture and disposal may add to pollution in the environment and the depletion of fossil fuels.
- Limited Heat Resistance: Polyethylene has limited heat resistance, and prolonged exposure to high temperatures can cause it to deform or melt, limiting its use in high-temperature applications.
Summary
In conclusion, because of its extraordinary qualities, polyethylene (PE) is a substance that is highly valued and finds extensive use in both industry and domestic settings. Due to its cost-effectiveness, durability, and adaptability, it is an essential component of contemporary consumer products manufacturing. PE is a favored material for a variety of applications in industry due to its strong chemical resistance and superior electrical insulating capabilities. It is an essential part of numerous packing materials, cable insulation, and pipelines. Its ease of molding and extrusion opens up a wide range of applications for complex designs and tailored solutions.
PE is an essential part of daily life in households. It offers ease and safety for many different objects, from necessary items like bottles to packaging materials like bags and containers. Its significance in improving the comfort and usefulness of living environments is highlighted by its application in flooring, furniture, and insulating materials. PE also guarantees hygienic practices and safety in applications related to medicine and personal hygiene.
It is imperative to acknowledge the environmental challenges linked to the manufacture and disposal of PE. Because it is a petroleum-based product, pollution, and resource consumption are increased during manufacture. To reduce its environmental impact, proper recycling and disposal procedures are essential.
References
- https://www.xometry.com/resources/materials/polyethylene/
- https://omnexus.specialchem.com/selection-guide/polyethylene-plastic
- https://byjus.com/chemistry/polyethylene/
- https://www.geeksforgeeks.org/polyethylene/
- https://testbook.com/chemistry/polyethylene
- https://www.toppr.com/guides/physics/atomic-and-molecular-structure/polyethylene-definition-and-properties/
- https://collegedunia.com/exams/polythene-chemistry-articleid-669
- https://induplast.es/en/b/readers-corner/about-plastics-information-and-interesting-facts/p/what-are-the-properties-of-polyethylene-pe-4-2
- https://www.intechopen.com/chapters/1157745
