Polystyrene is a polymer produced by the polymerization of styrene. It is commonly used in the food service industry for rigid trays and containers, disposable dining utensils, and foamed cups, plates, and bowls.
Styrene polymerizes to polystyrene using a typical free radical chain process. The polymerization process will begin with either heat or initiator. Thermal decomposition of initiators produces active free radicals, which initiate the polymerization process. Applications ranging from food packaging to electronics protection use polystyrene in a variety of forms, such as foam, film, and rigid plastic. While polystyrene has a wide range of applications and a moderate shrink rate, it also flows slowly and can become brittle.
Interesting Science Videos
What is polystyrene?
Polystyrene is a synthetic aromatic thermoplastic polymer derived from styrene monomers. Polystyrene can be transparent, rigid, and quite brittle. It is naturally transparent but can be colored by using colorants. Polystyrene is an odorless, and rigid thermoplastic.
It has good electrical characteristics and is easily molded. Its tensile strength is around 16 MPa. Its transparency makes it perfect for a wide range of household products, including toys and containers.
Polystyrene can easily copolymerize with monomers such as vinyl chloride, butadiene, acrylonitrile, and acrylates, resulting in unique characteristics. Modified polystyrene’s thermal and mechanical qualities enhance material elasticity and performance, making it widely used in industries.
Styrene homopolymers are sometimes known as general-purpose or crystal polystyrene. Because crystal polystyrene is brittle, styrene is typically polymerized with dissolved polybutadiene rubber to increase polymer strength. Such modified polystyrene is known as high-impact or rubber-modified polystyrene. High-impact polystyrene typically contains 88-97% styrene. Adding a blowing agent to polystyrene creates an expanded polystyrene.
Formation of polystyrene
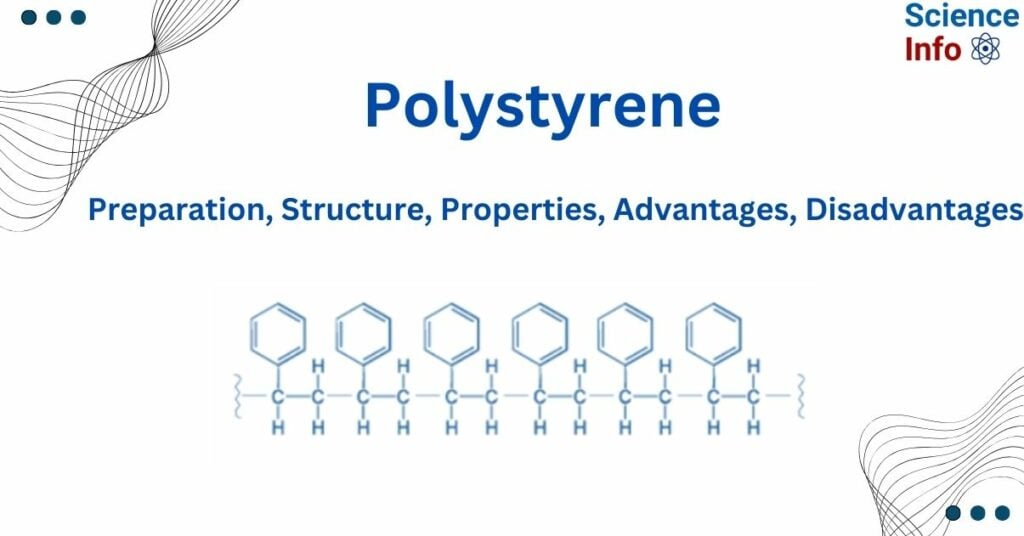
Styrene is polymerized using a free radical polymerization process to give polystyrene. Styrene, is a simple organic molecule, with the chemical formula C6H5CH=CH2. Styrene is sometimes called vinylbenzene. This is because it has a vinyl (-CH=CH2) functional group connected to a benzene ring (C6H6).
At 80-90 oC, the initiator benzoyl peroxide decomposes with the cleavage of an oxygen-oxygen link, resulting in two benzoyloxy radicals. These radicals then lose carbon dioxide, resulting in two benzyl radicals. Initiator radicals (R*) add to styrene’s C=C bond, creating a new benzyl-type free radical. The radical attaches to another styrene molecule, causing the polymer chain to extend. Polymer chain growth is eventually stopped by combining two radicals (either both polymer radicals or one polymer radical and one initiator radical).
Structure
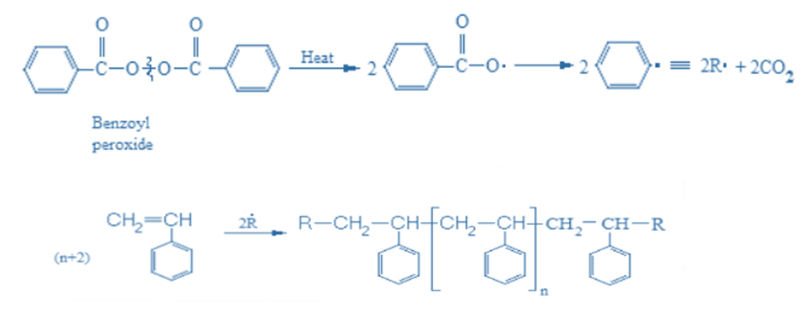
Polystyrene is a long-chain hydrocarbon with alternating carbon centers connected to phenyl groups, which are benzene derivatives. Polystyrene’s chemical formula is (C8H8)n, which comprises carbon and hydrogen.
Short-range van der Waals interactions among polymer chains affect the material’s characteristics. The large number of atoms in molecules creates a strong attraction between them. The chains can acquire a greater degree of confirmation and slide past one another when heated (or deformed quickly, as a result of a combination of viscoelastic and thermal insulating qualities).
Properties
- It is a hard and transparent thermoplastic.
- The density of polystyrene is 1.05 g/cm3.
- The boiling point of polystyrene is 430 degrees Celsius.
- Polystyrene’s thermal conductivity is 0.003 W/mK.
- Its specific gravity is 1.054.
- It has excellent optical properties, as it is a transparent polymer with strong transmission of all wavelengths.
- It is a lightweight substance, making it simple to handle and transport.
- Polystyrene has a low moisture absorption rate. It has high thermal insulation capabilities, making it ideal for situations where temperature control is crucial.
- It has excellent resistance to gamma radiation.
- It is chemically resistant, which makes it suitable for a wide range of applications.
- It is a non-biodegradable substance, with a few exceptions. Many aromatic hydrocarbon and chlorinated solvents dissolve it readily.
- The benzene ring’s chain stiffening effect causes polystyrene to be rigid but fragile. When dropped, it produces a distinctive metallic sound.
Applications
- It is used for manufacturing polystyrene items such as sheets, foam, brush handles, and combs.
- It is used to produce talcum powder.
- Refrigerators, air conditioners, ovens, microwaves, vacuum cleaners, blenders, and other appliances frequently employ polystyrene (solid and foam) since it is inert (does not react with other materials), inexpensive, and long-lasting.
- It is used to make automotive elements such as knobs, instrument panels, and sound-dampening foam.
- Polystyrene foodservice packaging keeps food fresher for longer and costs less than alternatives.
- Due to its affordable price, outstanding clarity, dimensional stability, and responsiveness to radiation sterilization, it is used for many medical applications. Its medical applications include sterilizing test tubes, diagnostic components, and other medical devices.
- It is often used in injection molding. Injection-molded polystyrene is commonly used for cosmetic and personal care containers, jewelry and photo equipment boxes, and photo film packaging.
Advantages
- It is cost-effective.
- It is resistant to many chemicals.
- It provides excellent thermal insulation.
- It can be molded into different sizes and shapes to use for various applications.
Disadvantages
- It is highly inflammable and is to be handled carefully.
- It is non-biodegradable and can remain in the environment for a long time.
- In its rigid form, it can be brittle and break easily under stress.
XPS and EPS
Extruded polystyrene (XPS) and expanded polystyrene (EPS) are two types of foam polystyrene that are utilized for insulation. Both are made of polystyrene resin. They have a closed-cell structure and utilize trapped air for long-term insulation.
Manufacturing procedures for EPS and XPS differ. EPS uses steam and the blowing agent pentane to expand polystyrene resin beads, which are then molded into blocks that can be cut to size. In contrast, XPS uses an extruder to process melted polystyrene resin and expand it using blowing agents.
- Both XPS and EPS foam have good thermal conductivity properties. However, the air trapped in the spaces of the EPS conducts heat. XPS foam offers a stable long-term thermal resistance, even in damp situations and at extremely cold temperatures.
- XPS foam is better suited to wetter settings that demand a higher water vapor diffusion resistance value. Moisture that penetrates small holes in EPS foam shrinks and expands. This has a substantial impact on its insulation performance while also causing premature deterioration. Closed-cell XPS foam repels water and can endure more than 1000 freeze/thaw cycles, offering long-term durability at a cheap cost.
- EPS is 10% to 30% cheaper than XPS per equal R-value and compressive strength.
SBS
Poly(styrene-butadiene-styrene), or SBS, is a strong rubber used for shoe soles, tire treads, and other applications that require durability. It’s a block copolymer. Its backbone chain is comprised of three fragments. The first is a long chain of polystyrene, the middle is a long chain of polybutadiene, and the last part is another long portion of polystyrene.
It is a type of thermoplastic elastomer that has the same elasticity as rubber at normal temperatures and can be melted to flow at high temperatures, similar to plastics, hence the name plastic material. Thus, the SBS-modified asphalt is distinguished by its lack of viscosity when heated and fragility when cooled, as well as its high flexibility and aging resistance.
The styrene-butadiene-styrene block copolymers with a polybutadiene content of up to 30wt%, known as crystal clear, impact-resistant, are fundamentally different from normal impact-resistant.
References
- https://byjus.com/chemistry/polystyrene/
- https://www.britannica.com/science/polystyrene
- https://www.sciencedirect.com/topics/chemical-engineering/polystyrene
- https://www.vedantu.com/chemistry/polystyrene
- https://ijcrt.org/papers/IJCRT2203531.pdf
- https://pslc.ws/macrog/sbs.htm
- https://www.sciencedirect.com/topics/engineering/styrene-butadiene-styrene#:~:text=Poly(styrene%2Dbutadiene%2Dstyrene,a%20long%20chain%20of%20polystyrene.
