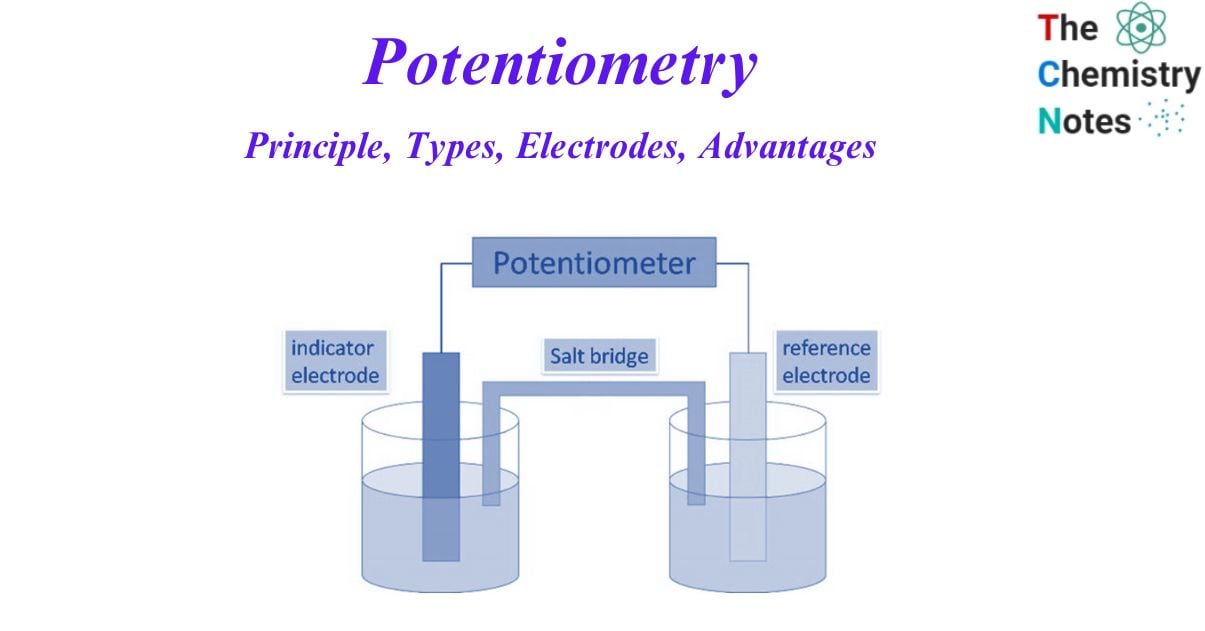
Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. The necessary equipment for potentiometry consists of a potential measurement instrument, a reference electrode, and an indication electrode.
Techniques known as potentiometric methods evaluate the electrical potential difference in an electrochemical cell and correlate it with the activity of the ionic components in the solution. A potentiometer, an indicator electrode, a reference electrode, and an electrolyte solution containing the ion are needed to perform a potentiometric measurement. Both electrodes are submerged in the solution and connected to a potentiometer. The electromotive force (emf), which is measured as the difference in potential between the two electrodes, is used in electrical engineering.
Interesting Science Videos
Principle of potentiometry
The potentiometry principle is based on the potential difference between the two electrodes being used. A change in the ionic concentration brought on by the addition of a titrant affects the potential difference. This potential difference is measured by the indicator electrode. After placing it in the sample solution, the reference electrode maintains its stability and potential value.
During potentiometric titration, the salt bridge is a divide that is used to prevent the interference of the analyte solution with the reference solution. Analyte solution is the solution whose potential we must determine.
The total electric potential or the potential difference can be calculated as follows:
Ecell = Eind – Eref + Ej
were,
Ecell = potential of the whole cell
Eind = potential of the indicator
Eref = potential or electromotive force of the reference electrode
Types of potentiometry
Direct potentiometry
Direct potentiometry is the measurement of an electrode potential directly from which the concentration of an active ion can be determined. Direct potentiometric measurements offer a quick and practical way to assess the activity of various cations and anions: a comparison between the potential that an indicator electrode develops in a cell while immersed in an analyte solution and that electrode’s potential when immersed in one or more standard solutions with a known analyte concentration.
Standard addition method
The standard-addition approach entails comparing the electrode system’s potential before and after a known amount of analyte solution is added in a measured volume of a standard.
Types of electrodes used in potentiometry
Indicator electrodes
The ideal indicator electrode should react quickly to changes in ion concentration as well as when other ions present in the sample matrix are present. Consequently, a perfect indicator electrode is selective, repeatable, and functional for a long period. The ion-selective electrode is the most effective indicator electrode in potentiometry. There are mainly two types of indicator electrodes. They are:
Metallic indicator electrode
These electrodes develop electric potential in response to redox reactions on the metal surface. It responds to a redox reaction at the metal surface and does not participate in many chemical reactions (Inert). It simply transmits e- to or from a reactive species in solution and works best when its surface is large and clean. Au, Pt, Ag, Cu, Zn, Cd, and Hg can be used as indicator electrodes.
These are mainly classified into four types of electrodes used in potentiometry.
I. First type of electrode
A simple metal electrode of the first type is submerged in a solution that contains its ion. These electrodes are reversible concerning their metal ion, implying that they are in direct equilibrium with the solution.
e.g., if we place a copper metal in a solution containing metal ion Cu2+ then,
Half cell reaction is: Cu2+ + 2e- → Cu (s)
Electrode potential by the activity Of Cu2+ is
E ind = E0 Cu –(0.0592/2) log a Cu(s) / a Cu2+
The first type of electrode is not common because…
- Many metal electrodes dissolve in the presence of acids, therefore they can only be used in neutral or basic solutions.
- Easily oxidized; only usable after deaeration to eliminate oxygen from analyte solutions
- Some metals don’t offer reproducible potentials.
- Metallic indicator electrodes are not very selective and react to both their cations and other cations that can be decreased more quickly.
Limitation:
- Interferes with cu+2 in a less selective manner than Ag+.
- May depend on pH.
- Quickly oxidized; deaeration necessary.
- An inconsistent response.
2. Second kind electrode
The activity of the metal ion or anion employed in the coating can be directly determined using this kind of electrode.e.g., if we use a silver electrode for chloride ion determination.
Half cell reaction is
AgCl(s) + e- Ag(s) + Cl-
Electrode potential is E, nd = EO — (0.0592/1) log a Ag (s) acr /aAgCI(s)
3. Third kind electrode:
It is a metal electrode assembly, and the equilibrium potential depends on the concentration of a cation present in the solution that is distinct from the cation of the electrode metal.
a mercury electrode, for instance, is used to measure the pH of calcium-containing liquids. A new equilibrium is created if a little amount of a solution containing the calcium EDTA complex is also added.
Equilibrium reaction is
CaY2- Ca2+ + Y 4-
The potential of a mercury electrode in this solution is
E ind = K – (0 05920) log . Kf a CaY2- /aca2+
4. Metallic redox indicator
Inert metal electrodes (Pt, Au, Pd) are frequently used as indicator electrodes in oxidation-reduction systems. The electrode serves as an e-source/sink for electrons moved from the solution’s redox system.
e.g., Detection Of Ce3+ with Pt electrode
Half-cell reaction is
Ce4+ + e- Ce3+
Potential is
E ind = EO – (0.0592/1) log a Ce3+/a Ce4+
Limitation:
- Processes of electron transport at inert electrodes are typically irreversible.
- It does not react to table reactions predictably.
Ion selective electrode
An ion-selective electrode (ISE) is an electrochemical sensor that operates on the idea of potentiometry, or measuring the cell potential (i.e., ISE versus a standard reference electrode) at near-zero current. In these circumstances, the boundary potential at the ISE-solution interface is controlled by electrochemical thermodynamic rules or by the well-known Nernst equation.
Potentiometric sensors known as ion-selective electrodes (ISEs) have a selective membrane to reduce matrix interferences. The pH electrode, which has a thin glass membrane that reacts to the H+ content in a solution, is the most used ISE. Fluoride, bromide, nitrate, cadmium, and gases in solution such as ammonia, carbon dioxide, nitrogen oxide, and oxygen are other characteristics that can be assessed.
Glass electrode
In a glass electrode, the sensing material is a thin glass membrane in the form of a bulb. Hydrogen ions in the fluid can make it sensitive. A potential is created in a thin glass barrier that separates two electrolytes.
A pH-sensitive glass electrode is a carbon dioxide electrode. It is used for the determination of dissolved carbon dioxide in water samples and is highly helpful in environmental chemistry.
Ion exchange membrane
Ion exchange electrodes are built on a membrane that conducts electricity. A form of organic polymer or ion-exchange resin called an ion-exchange membrane, carries particular dissolved ions out of the solution while blocking other ions or neutral molecules.
To move H+ ions, proton-exchange membranes are employed.
In some alkaline fuel cells, OH ions from dissolved solutions are transported using anion exchange membranes.
Enzyme electrode
Enzyme electrodes are based on the idea that substrates and enzymes can interact during biological processes. Clark and Lyons were the first to develop it.
By keeping β-glucosides in a gel layer and connecting a cyanide-sensitive membrane electrode to it, it is possible to create an amygdalin-sensitive electrode. Enzyme electrodes can monitor glucose and urea. Patients who have diabetes can benefit from it.
Crystalline electrode
Electrodes made of crystalline or solid-state membranes include an insoluble inorganic salt. Electrodes made of crystal offer high selectivity. The electrode response may be affected by ions that can enter the crystal structure. The potential is created at the membrane of crystalline electrodes through an ion-exchange mechanism.
Reference electrode
A reference electrode is stable and has a known electrode potential. Its remarkable stability is accomplished by using the redox system, which requires saturated concentrations in each of the participating solutions of the reaction.
Reference electrodes need to have a repeatable and steady voltage. Preferably, reversible-type electrodes are employed as reference electrodes. A tiny cathodic current in a reversible electrode causes the reduction reaction, whereas a small anodic current causes the oxidation reaction.
Silver/Silver Chloride Electrode
The silver/silver chloride reference electrode is made of a silver wire (Ag) coated with a coating of solid silver chloride (AgCl), which is then submerged in a solution that is saturated with KCI and AgCl.
The reaction in the half cell is AgCl (s) + e – Ag (s) + CI (satd).
Advantages
- Easy to produce
- 2. Rapidly demonstrates potential and achieves reproducible equilibrium between -300C and 1350C.
- Stable & accurate despite large temperature variations
Disadvantages
- It reacts to samples.
Saturated calomel electrode
A reference electrode based on the reaction between elemental mercury and mercury (I) chloride is known as a saturated calomel electrode (SCE). A saturated solution of potassium chloride in water serves as the aqueous phase in contact with the mercury and mercury (I) chloride. The electrode is generally connected to the fluid in which the other electrode is immersed via a porous frit ie., salt bridge.
Advantages
- The entire pH range
- Compared to a silver electrode, mercury ions react with fewer samples.
- Reliable electrode
Disadvantages
- A high-temperature coefficient
- Establishment takes longer
- The chloride ion solution exhibits incompatibility.
Reference hydrogen electrode
The hydrogen electrode serves as a reference for electrode potential measurements. Theoretically, it is the most significant electrode for usage in aqueous solutions. In a solution of hydrogen ions with unit activity, the reversible hydrogen electrode displays a potential that is thought to be zero at all temperatures.
Salt bridge
A salt bridge (also known as an ion bridge) joins the galvanic cell’s oxidation and reduction half cells. As a result, the two half-cells of a galvanic cell continue to be neutral.
Galvanic cells are made up of two halves oxidation and reduction. Charges begin to accumulate at anodes and cathodes as the electrochemical reaction progresses due to charge transfer.
The salt bridge minimizes or inhibits the potential at the liquid-liquid interface and prevents mechanical flow or diffusion of a solution from one-half cell to the other.
Liquid junction potential
Ions diffuse from higher to lower concentrations when two electrode solutions with different concentrations are brought close together. The speed of the ions in the electric field and the rate of diffusion are approximately equal. Therefore, if the cation moves quicker, the cation diffuses in the diluted solution more quickly than the anion and the diluted solution acquires a positive charge. Similarly, if an anion moves quicker, the dilute solution becomes negatively charged. In either scenario, a potential difference develops at the junction of two solutions due to the formation of an electrical double layer.
Liquid junction potential is the term used to describe the potential difference that forms at the intersection of two solutions with different concentrations. The magnitude of the liquid junction potential is dependent on the relative speeds of the two ions and results from the differing migrational velocities of the two ions. There won’t be any liquid connection potential if the two ions move at the same speed.
Boundary potential
The boundary potential is located on the glass membrane’s surface, at the contact between the hydrated gel layer and the external solution. The activity of hydrogen ions in the external solution and the activity of hydrogen ions on the surface of the gel both have a role in the boundary potential that develops when the electrode is submerged in an aqueous solution. The ions will typically move in the direction of lower activity, much like at a liquid junction, which is one explanation for the potential. As a result, a tiny layer of charge that serves as a potential is built up on the membrane’s surface.
Diffusion potential
The protons in the inner gel layer tend to diffuse towards the dry membrane, which includes —SiO–Na+, while sodium ions in the dry membrane tend to migrate towards the hydrated layer, which results in the diffusion potential.
A particular kind of liquid-junction potential is produced by the ions’ various rates of ion migration. However, the same event occurs in the reverse way on the other side of the membrane. Since they effectively cancel one another, the net diffusion potential is relatively small, and the boundary potential substantially determines the membrane’s potential.
Applications of potentiometry
- ISEs, or ion-selective electrodes react only to a certain ion in solution. To determine the concentration of particular ions in a solution, potentiometry is frequently used with ISEs.
- The concentration of medicines in biological fluids like blood and urine can be detected using potentiometry.
- Redox titrations can also be carried out using potentiometry. This involves determining the potential difference that occurs during the titration between the working electrode and the reference electrode. When the analyte has fully oxidized or reduced, the potential difference can be used to calculate the titration’s endpoint.
- Potentiometry can be used to check the soil’s and water’s quality.
- To estimate the pH of a solution, potentiometry is frequently employed in acid-base titration. The potential difference between the sample and the reference electrode is measured via this method using a pH electrode.
- Potentiometry is used to measure the salt content of meat, fish, dairy products, fruit juices, and brewing solutions as well as the corrosive effects of NO3 on canned foods, Ca in the production of dairy products and beer, K in the production of fruit juice and wine.
- Potentiometry is employed in agriculture to identify various elements in soils, fertilizers, etc.
- Potentiometry is used to analyze cyanide, ammonia, and other substances found in water or wastewater.
- Potentiometry is employed in the production of detergents, food processing, etc.
References
- https://www.bdn.go.th/tp/ebook/qQEcAat1pR9gC3q0GT5gMJq0qT5co3uw.
- https://www.vedantu.com/chemistry/potentiometric-titration.
- https://unacademy.com/content/nta-ugc/study-material/pharmaceutical-analysis/theory-and-principles-of-potentiometry/.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2515866/,
- http://rxpharmaworld.blogspot.com/2016/12/potentiometry.html
- https://faculty.ksu.edu.sa/sites/default/files/Chapter%2021%20%281%29.pdf
