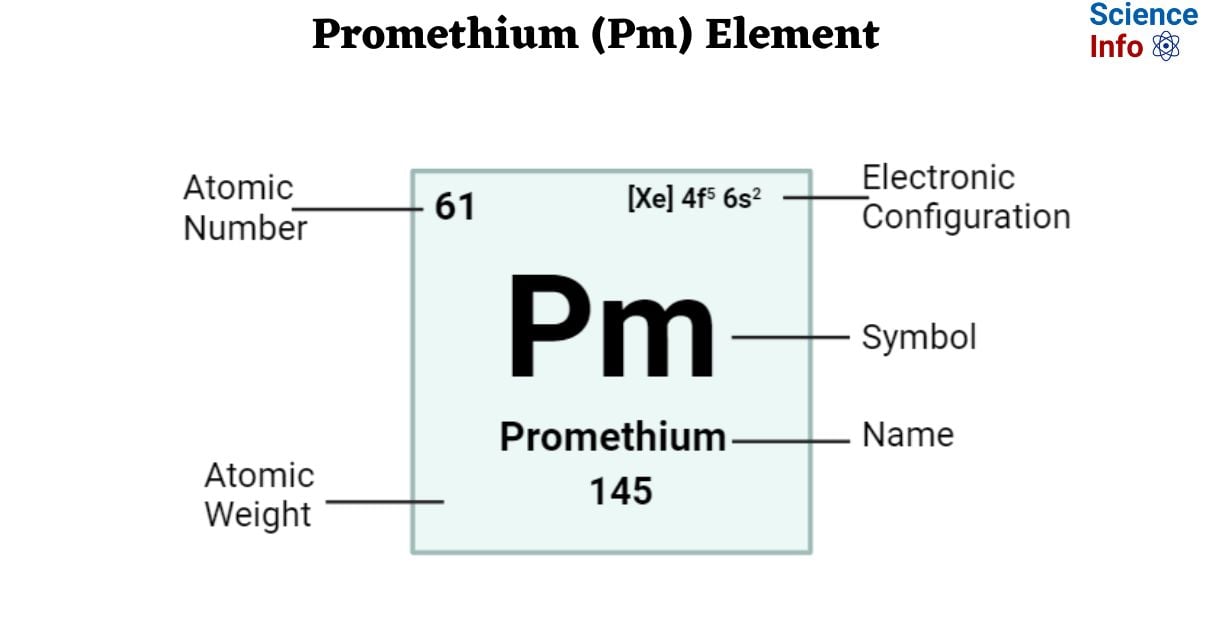
Promethium is a chemical element with the atomic number 61 and is represented by the symbol ‘Pm’ in the periodic table. It is soft and silvery in appearance classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. All isotopes of this element exhibit radioactive properties, rendering them unstable. Moreover, this element is marked for its scarcity, as it is found in minute quantities of approximately 500-600 grams within the Earth’s crust at any given moment.
Interesting Science Videos
History of Promethium
- The initial anticipation of the presence of an element positioned between neodymium (atomic number 60) and samarium (atomic number 62) was postulated by Bohuslav Brauner, a Czech chemist, in the year 1902.
- The element in question was purportedly discovered by Luigi Rolla, an esteemed Italian chemist affiliated with the Royal University in Florence. Although his findings were not officially published until 1926, Rolla claimed to have made this discovery in 1924. In accordance with his association with the university, he bestowed upon the element the name “florentium.”
- In 1945, Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell successfully isolated element 61, providing conclusive evidence. This achievement took place at Oak Ridge, Tennessee, where they obtained the element from the fission products of uranium. Promethium-147 was successfully isolated through the utilization of ion exchange chromatography.
- The element promethium was officially designated with its name in 1949 by the International Union of Pure and Applied Chemistry (IUPAC).
- The origin of the term can be traced back to Prometheus, a prominent figure in Greek mythology who is said to have stolen fire from Mount Olympus and given it to humanity.
Occurrence of Promethium
- Promethium has not been detected in significant quantities within the terrestrial lithosphere. Trace amounts of this substance are present in uranium ores as a byproduct of uranium decay. Promethium can be generated as a byproduct of uranium fission.
- Promethium exhibits 29 isotopes, each possessing distinct half-lives, spanning a range of mass numbers from 130Pm to 158Pm. Promethium does not possess any isotopes that occur naturally.
- Additionally, the production of this substance can be achieved through the process of bombarding neodymium-146 with neutrons. The isotope neodymium-146 undergoes a neutron capture reaction, resulting in the transformation of the nucleus into neodymium-147.
- The isotope of promethium that exhibits the highest stability, known as promethium-145, possesses a half-life of approximately 17.7 years. The process of electron capture leads to the decay of the given element, resulting in the formation of neodymium-145.
Elemental Properties of Promethium
| Electronic Configuration | [Xe] 4f5 6s2 |
| Atomic Number | 61 |
| Atomic Weight | 145 g.mol -1, no stable isotope |
| State at 20°C | Solid |
| Group, Period, and Block | lanthanide, 6, f-block |
| Density | 7.26 g.cm -3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 23, 8, 2 |
| Electrons | 61 |
| Protons | 61 |
| Neutrons in most abundant isotope | 84 |
Physical Properties of Promethium

- Promethium has an atomic number of 61 and is a silvery-white rare earth metal. It has a melting point of 1042°C (1908°F) and a boiling point of 3000°C (5432°F).
- Pm has a solid phase density of 7.26 g/cm3.
- Promethium exhibits a double-hexagonal close-packed alpha structure at temperatures lower than 890°C. At temperatures exceeding 890°C, promethium exhibits a body-centered-cubic beta phase.
- 145Pm is the most stable radioactive isotope of the chemical element promethium, characterized by a half-life of approximately 18 years, specifically 17.7 years.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1315 K (1042 °C, 1908 °F) |
| Boiling point | 3273 K (3000 °C, 5432 °F) |
| Density | 6.475 g.cm-3 at 20° |
| Malleability | n/a |
| Ductility | n/a |
| Electronegativity | 1.13 (Pauling Scale) |
Chemical properties of Promethium
- Promethium compounds are known to exist solely in the +3 oxidation state.
- It has been duly observed that compounds with promethium exhibit a distinct chromatic manifestation, typically presenting as a vibrant hue of pink or red.
- Promethium compounds demonstrate a tendency for dissolution when exposed to hydrochloric acid, leading to the creation of trichloride. Likewise, upon exposure to nitric acid, these compounds exhibit the inherent ability to produce the corresponding nitrate compound.
Uses of Promethium
Promethium exhibits a rather constrained range of applications. The uses of promethium are listed here:
Used As Luminescent
Certain compounds containing promethium exhibit luminescent properties. Luminescence is a characteristic wherein light emission occurs without concurrent thermal emission. The emission of light by a firefly serves as an illustration of luminescence. Promethium compounds exhibit luminescence due to the emission of radiation. Certain signal lights employ a luminescent coating infused with phosphor. The phosphor material effectively captures and assimilates beta radiation emanating from the promethium-147 isotope, subsequently manifesting a discernible chromatic emission.
Used In Batteries
Promethium possesses the potential to serve as a viable energy source. The emission of radiation by promethium yields a form of energy akin to that which is derived from a conventional electrochemical cell commonly referred to as a battery. The utilization of a promethium battery is particularly advantageous in scenarios where alternative battery types would prove excessively burdensome or voluminous for implementation, such as in the context of satellites or space probes. These batteries are prohibitively costly for widespread utilization, nonetheless.
Used For Detecting Thickness
Promethium measures material thickness. Imagine a conveyor belt producing thin metal sheets. A detector is below a promethium metal sample. The detector measures metal radiation. Radiation passes less through thick metal sheets. Radiation penetrates thinner sheets. The detector indicates metal thickness. This stops the conveyor belt automatically.
Health Effects of Promethium
Promethium lacks any apparent biological relevance and is considered to be moderately harmful owing to its significant radioactivity. The animal-based experimental trials provided evidence that the substance displayed a propensity for specific localization on the outer surface of bones, resulting in a protracted elimination process.
Environmental Effects of Promethium
Promethium is exceedingly scarce in its natural occurrence, thereby rendering it inconsequential in terms of environmental impact. Due to its significant radioactivity, careful handling is required.
Video on Promethium
References
- https://www.rsc.org/periodic-table/element/61/promethium
- https://pubchem.ncbi.nlm.nih.gov/element/Promethium
- https://www.britannica.com/science/promethium
- https://education.jlab.org/itselemental/ele061.html
- https://www.lenntech.com/periodic/elements/pm.htm
- https://byjus.com/chemistry/promethium/

