A polyatomic ion’s or molecule’s electron delocalization is described by resonance structures. Due to the presence of partial charges and fractional bonds in a molecule/polyatomic ion, a single Lewis structure fails to describe the bonding in many circumstances. Resonance structures are utilized to characterize chemical bonding in such circumstances.
Interesting Science Videos
What is Resonance?
The process of characterizing bonds in polyatomic ions and some molecules after combining various contributing structures is known as resonance. Several contributing components or structures play an important part in the resonance of a structure; they are also known as canonical or resonance structures. They are important in chemistry because they help us comprehend the structures of many compounds better. They are commonly used to describe chemical bonding between elements in the valence bond hypothesis.
Significance of Resonance in Chemistry
- Resonance is an important organic chemistry phenomena that aids in the understanding of the diverse chemical structures of compounds, radicals, ions, and so on.
- It makes the chemical structures of certain compounds more stable by shifting the positions of their double bonds. It may be found in a variety of chemicals and molecules, including benzene, nitrobenzene, and others.
- It aids in explaining the reactivity of the chemicals. The greater a compound’s stability, the less reactive it is in nature. Both of these qualities have an inverse proportionality relationship.
Things To Consider While Drawing Resonance Structure
Here are a few things to consider while drawing resonance structures:
- Never move the atoms; only the electrons should be moved: This contains lone pair electrons and pi bonds (remember, a double bond has one pi bond and a triple bond has two pi bonds).
- A single bond (also known as a sigma bond) should never be broken.
- Work in only one area at a time: start with the three atoms closest together and work your way along the molecule.
- You should start with electron-dense areas: This implies you’ll be moving electrons from the negative to the positive charge.
- In most cases, you’ll be converting lone pairs into bonds and bonds into lone pairs.
- Follow the octet rule and avoid high formal charges.
How to Draw Resonance Structure
- They must have the same number of electrons.
- They have to be Lewis dot structures.
- The hybridization between the structures remains unchanged.
- The molecules are only moved by electrons. The atoms stay in their original locations.
- The most important and contributory resonance structure may be identified using the formal charges of each atom.
- Structures with fewer formal charges are more stable than those with many. Charged structures are less important than neutral ones.
- Structures with a complete octet are more significant than those with an incomplete octet.
Resonance Structure Examples
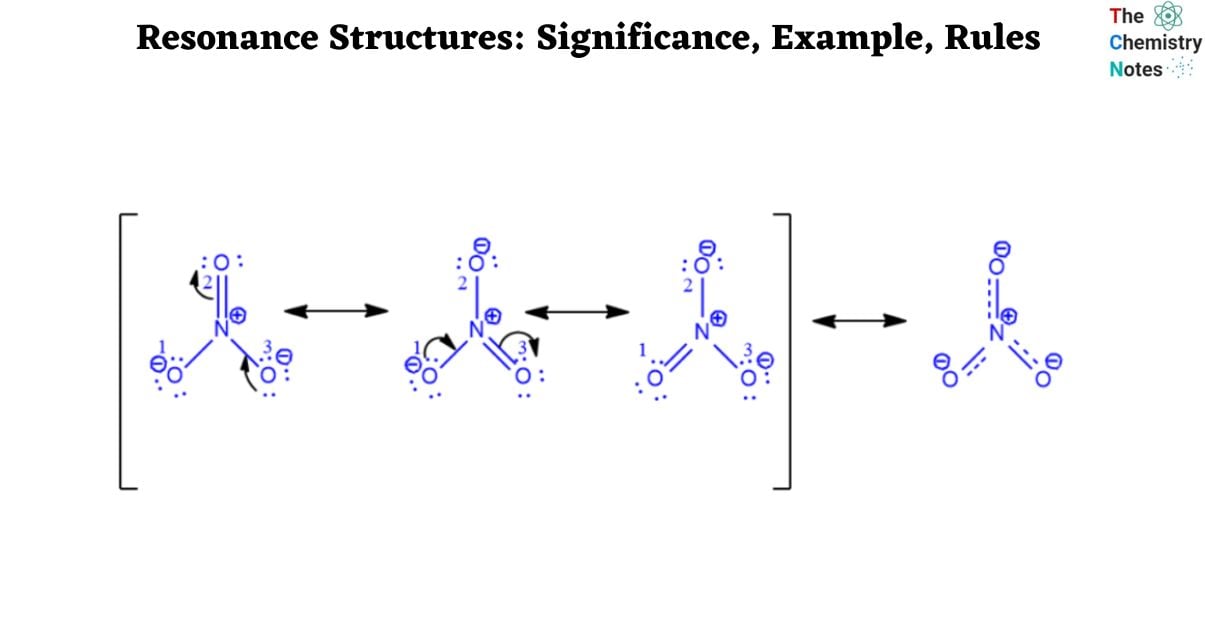
Nitrate Ion (NO3)–
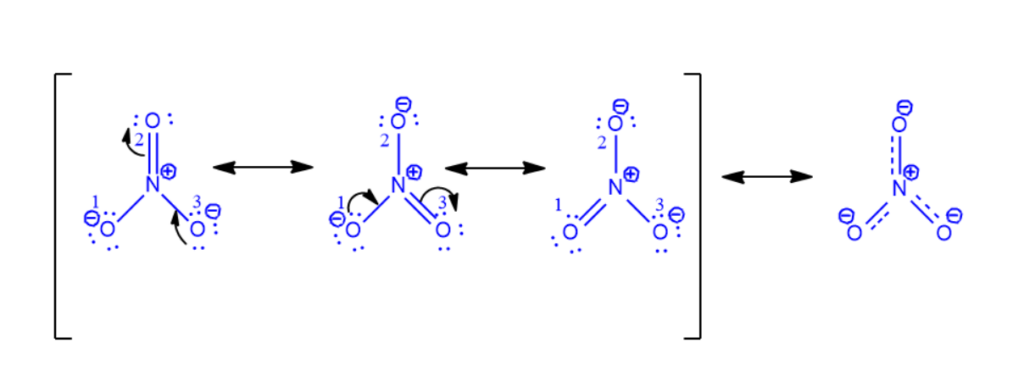
Step 1: Draw the NO3– Lewis dot structure, as seen below.
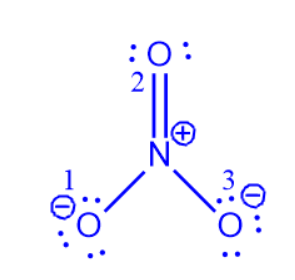
NO3– contains two oxygen atoms (O1 and O3) with three lone pairs, as seen in the figure. Furthermore, these two oxygen atoms have a negative charge and are singly linked to the nitrogen atom. Another oxygen atom (O2) is doubly linked to nitrogen and has no charge. Nitrogen has no lone pairs and a charge of +1. The octet rule applies to all nitrogen and oxygen atoms.
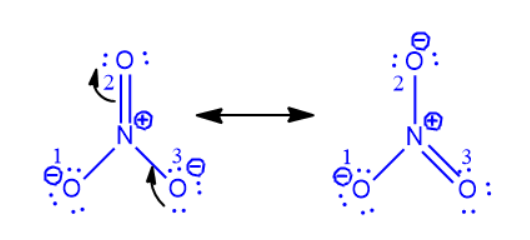
Step 2: Transform one lone pair of charged oxygen (O3) atoms into a nitrogen bond. With nitrogen having 10 electrons in its valence shell, the donor atom turns neutral and forms a new pi bond with it. It won’t break the rule of octets.
Nonetheless, nitrogen and oxygen (O2) form another pi bond. The electrons will go to the oxygen atom upon the breakage of this pi link. Nitrogen currently possesses eight electrons. O2 will be negatively charged since it gains a lone pair. As a result, one oxygen atom (O3) gradually gains a negative charge from another (O2). Use a curved arrow to represent the electron shift, as in the illustration below.
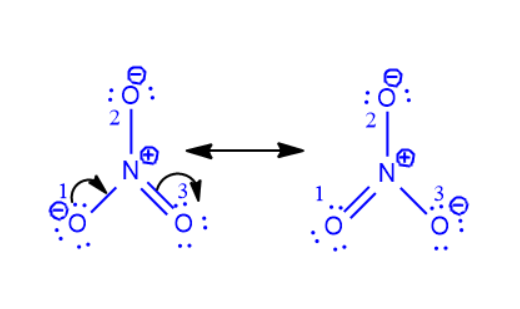
Step 3: Step 3 is a continuation of Step 2 in which a pi bond is formed with nitrogen by the other charged oxygen atom (O1) giving up its lone pair. The procedure is given below.
In actuality, none of the three resonance structures exist at the same time. Below are the resonance hybrid and resonance structures of NO3–.
What is the most stable resonance structure?
The most stable resonance structures are those that have the least amount of energy. This is important since a single structure will contribute more to the resonance hybrid the more stable it is. The primary contributors are those structures that are the most stable. In the meanwhile, the structures that are least stable are referred to as minor contributors. Five key guidelines need to be followed in order to determine which resonance structure is the most stable. (Note: two equal structures will provide an equal contribution.)
Resonance Stability Rules:
- Compared to single structures, the resonance hybrid exhibits greater stability.
- Of all the structures, the one without any charges is the most stable.
- In comparison to structures with more formal charges, those with less formal charges are more stable.
- The most electronegative atom will have the negative charge in the most stable configuration.
- The least electronegative atom will have the positive charge in the most stable configuration.
Video on Resonance Structures
References
- https://www.chemistrylearner.com/resonance-structures.html
- https://pediaa.com/how-to-draw-resonance-structures/#google_vignette
- https://courses.lumenlearning.com/suny-mcc-organicchemistry/chapter/how-to-draw-resonance-contributors/
- https://byjus.com/chemistry/resonancestructures/#:~:text=Resonance%20structures%20are%20sets%20of,and%20fractional%20bonds%20in%20it.
- https://chemistrytalk.org/resonance-structures/
- https://www.chem.ucalgary.ca/courses/353/Carey5th/Ch01/ch1-7.html

