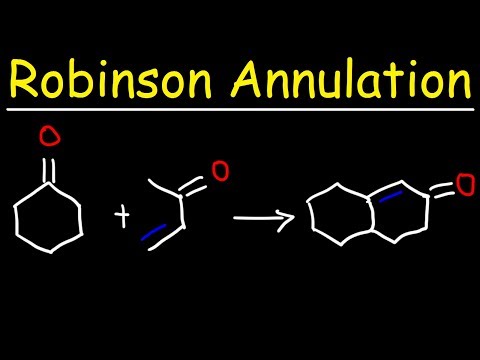
The Robinson annulation reaction is used to synthesize polycyclic compounds. A reaction that adds a new ring to a molecule is known as an annulation reaction because the word annulation comes from the Latin annulus, which means “ring.”
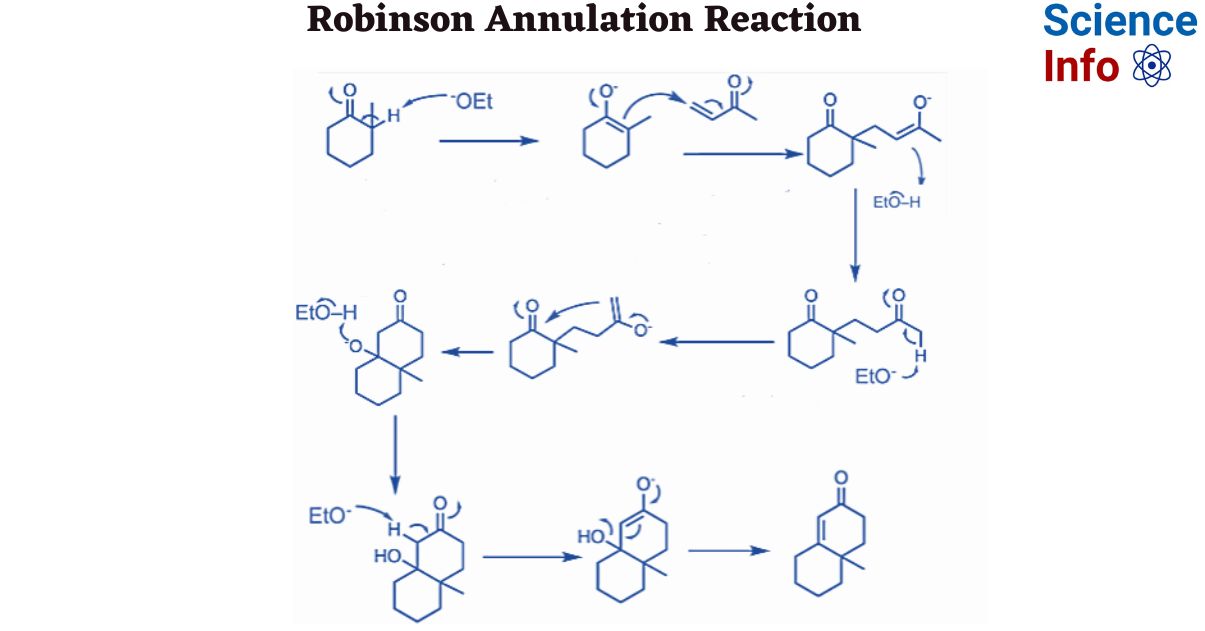
The Robinson annulation, named for British scientist Robert Robinson who won the 1947 Nobel Prize, is a two-step procedure that combines an intramolecular aldol reaction with a Michael reaction.
Interesting Science Videos
What is Robinson Annulation Reaction?
The Robinson Annulation is a method for forming a six-membered ring from alpha,beta-unsaturated ketone that combines two previously known processes, the Michael addition and intramolecular aldol condensation.
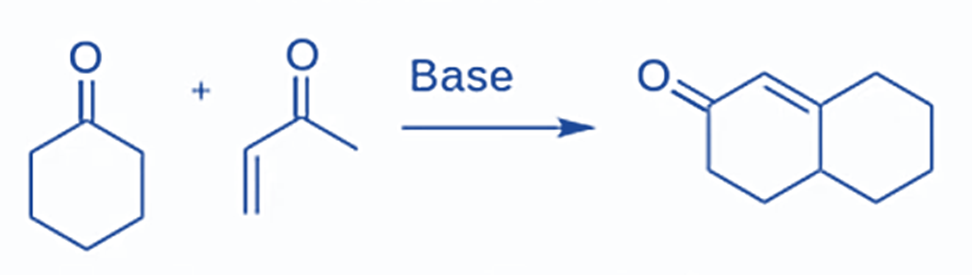
The starting parts are a ketone and an alpha,beta-unsaturated ketone, which is a ketone with an alkene located only one carbon away. The next steps will now use a Michael Addition to unite the two distinct molecules into a single one, and an intramolecular aldol condensation will react the two ends of this single molecule with one another to close the ring. The ultimate result of the two reactions is a 6-membered ring containing one alpha,beta-unsaturated ketone.
Robinson Annulation Reaction Mechanism
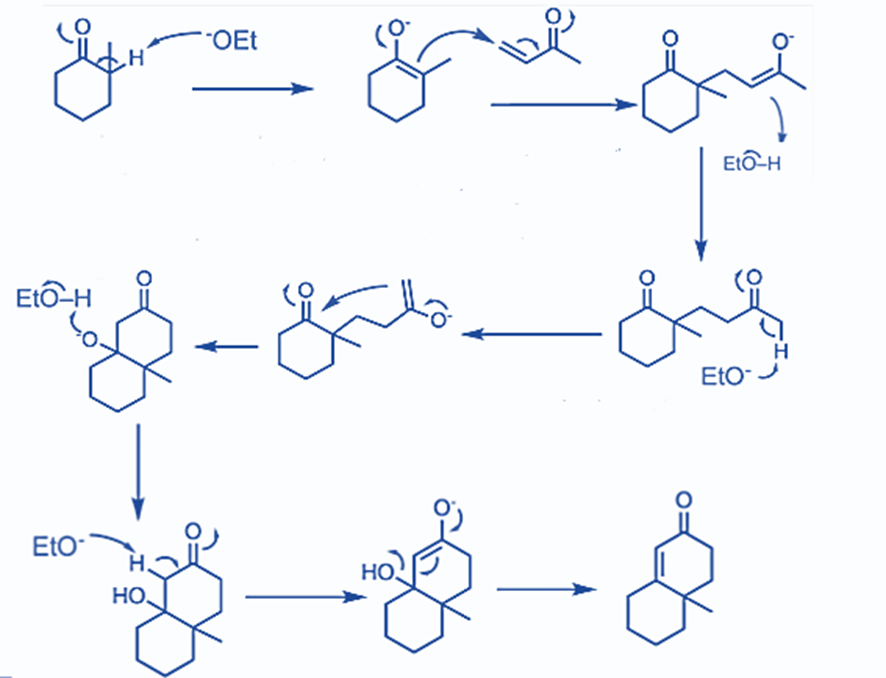
Step 1: Michael addition to an α,β-unsaturated ketone occurs in the first phase. To facilitate further reaction, tautomerization occurs and enolate is produced.
Step 2: In this step, cyclization occurs. The six-membered cyclic product is formed via the aldol condensation reaction. α,β-unsaturated cyclic ketones are formed as a result of further hydrolysis.
Application of Robinson Annulation Reaction
- The synthesis of spirocyclic molecules also employs Robinson annulation.
- The Robinson Annulation is still the preferred method for synthesizing naturally occurring steroids, alkaloids, and terpenoids because of its versatility in the complete synthesis of complex compounds.
- Robinson annulation was utilized by F. Dean Toste and coworkers in the complete synthesis of (+)-fawcettimine, a tetracyclic Lycopodium alkaloid with possible value in acetylcholine esterase inhibition.
- Robinson Annulation is regarded as a formal [4+2] condensation reaction, meaning that the reaction always results in the formation of new rings with six members.
- The Robinson annulation product of 2-methyl-cyclohexane-1,3-dione and methyl vinyl ketone is the Wieland-Miescher ketone. This chemical is utilized to synthesize steroids with key biological characteristics and can be made enantiopure using proline catalysis.
- The definition of Robinson annulations has been expanded to include [3+3] annulations. Robinson annulation is the broad term used to describe all ring-forming cascades involving the sequence of Michael and intramolecular aldol reaction.
- The Robinson annulation is the pivot point for countless syntheses. Those that use the Robinson annulation to create an internal ring are noteworthy. A synthetic pathway is often made simpler when a functionalized chain is present at the vinyl ketone’s alpha carbon. A very significant synthesis of the six-membered ring is Robinson Annulation.
Video on Robinson Annulation Reaction
References
- https://byjus.com/chemistry/robinson-annulation/
- https://www.masterorganicchemistry.com/2018/12/10/the-robinson-annulation/
- https://ncstate.pressbooks.pub/organicchem/chapter/the-robinson-annulation-reaction/
- https://unacademy.com/content/nta-ugc/study-material/chemistry/robinson-annulation-reaction/
- https://chemistrytalk.org/robinson-annulation/