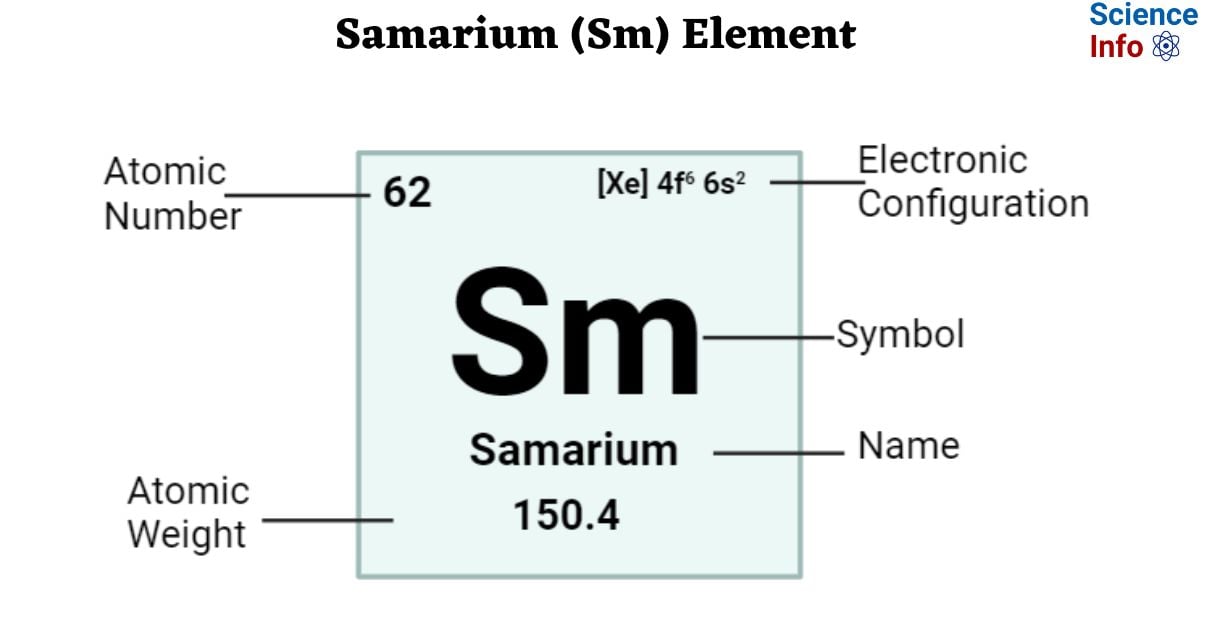
Samarium is a chemical element with an atomic number 62 and is represented by the symbol ‘Sm’ in the periodic table. It is soft and silvery-white in appearance classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Samarium generally has an oxidation state of +3.
Samarium is positioned as the 40th most abundant element in the Earth’s crust, exhibiting a higher occurrence than specific metals such as tin. It can be observed in diverse minerals, including cerite, gadolinite, samarskite, monazite, and bastnäsite, with concentrations reaching up to 2.8%. Monazite and bastnäsite are commonly employed as primary sources of the element in various commercial applications.
Interesting Science Videos
History of Samarium
The spectroscopic observation of samarium was conducted by Jean Charles Galissard de Marignac, a Swiss chemist, in the year 1853. This observation was made in a substance called dydimia.
In the year 1879, the chemist Paul-Émile Lecoq de Boisbaudran, who originated from France, achieved the successful utilization of the mineral samarskite ((Y, Ce, U, Fe)3(Nb, Ta, Ti)5O16) in order to isolate samarium, marking the first instance of such isolation.
Occurrence of Samarium
- Samarium is ranked as the 40th most prevalent element in the Earth’s crust, surpassing the occurrence of certain metals like tin.
- Samarium is not naturally occurring in its elemental form but instead exists within various minerals, predominantly monazite, and bastnaesite.
- The presence of the element can be detected in various minerals such as cerite, gadolinite, samarskite, monazite, and bastnäsite, with concentrations that may reach up to 2.8%.
- Samarium metal can be generated through the process of electrolysis, wherein the molten chloride is subjected to the presence of sodium chloride.
- Samarium exhibits a total of 30 isotopes, spanning mass numbers 131Sm to 160Sm, for which their respective half-lives have been determined.
Isotopes of Samarium
Naturally occurring samarium consists of seven isotopes: 144Sm (3.1%), 147Sm (15.0%), 148Sm (11.2%), 149Sm (13.8%), 150Sm (7.4%), 152Sm (26.7%), and 154Sm (22.8%).
Naturally Occurring Isotopes of Samarium
| Isotopes | Natural Abundance (atom %) |
|---|---|
| 144Sm | 3.1 |
| 147Sm | 15.0 |
| 148Sm | 11.2 |
| 149Sm | 13.8 |
| 150Sm | 7.4 |
| 152Sm | 26.7 |
| 154Sm | 22.8 |
Elemental Properties of Samarium
| Electronic Configuration | [Xe] 4f6 6s2 |
| Atomic Number | 62 |
| Atomic Weight | 150.4 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Lanthanides, 6, f-block |
| Density | 7.54 g/cm3 g.cm -3 at 20 °C |
| Appearance | silvery-white |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 24, 8, 2 |
| Electrons | 62 |
| Protons | 62 |
| Neutrons in the most abundant isotope | 90 |
Physical Properties of Samarium
- Samarium has an atomic number of 62 and is a silvery-white rare earth metal. It has a melting point of 1072°C (1962°F) and a boiling point of 1900°C (3450°F).
- Samarium has a solid phase density of 7.52 g/cm3 and a liquid or molten phase density of 7.16 g/cm3.
- Samarium exhibits the highest level of hardness and brittleness among the rare earth elements.
- Samarium is considered to be the third most volatile lanthanide element, following ytterbium and europium.
- Under normal environmental conditions, samarium exhibits a rhombohedral alpha-form structure.
- At a temperature of 731°C, a transition in the crystal structure occurs, leading to the adoption of a hexagonally close-packed arrangement. At a temperature of 922°C, the substance undergoes a phase transition and adopts a body-centered cubic crystal structure.
- Samarium exhibits paramagnetic properties at standard room temperature but undergoes a transition to an antiferromagnetic state upon cooling to a temperature of -258.35°C.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1345 K (1072 °C, 1962 °F) |
| Boiling point | 2173 K (1900 °C, 3452 °F) |
| Density | 7.52 g cm-3 at 20° |
| Malleability | No |
| Ductility | No |
| Electronegativity | 1.17 (Pauling Scale) |
Chemical Properties of Samarium
- Samarium exhibits stability in ambient air conditions, yet it undergoes combustion in the presence of air when subjected to temperatures exceeding 150°C. When exposed to humid air, it undergoes oxidation, resulting in tarnishing.
- Samarium undergoes a gradual oxidation process when exposed to ambient conditions. Despite being immersed in mineral oil, a gradual oxidation process is observed. In order to prevent oxidation, it is necessary to store it within an environment that is devoid of reactive substances.
- The reaction between samarium metal and water results in the formation of a hydroxide compound. The reaction rate of this substance is relatively sluggish when exposed to cold water, but significantly accelerates when subjected to hot water. The rate of a chemical reaction is observed to exhibit a positive correlation with the temperature of the water in which it takes place.
- Samarium typically assumes a trivalent ion state, denoted as Sm3+, in its chemical compounds. The majority of its salts exhibit a pale yellow hue.
- Samarium demonstrates a notable degree of solubility in sulfuric acid, leading to the creation of compounds that display a range of colors, ranging from yellow to pale green.
- Samarium has the ability to react with various elements, resulting in the formation of oxides, chalcogenides, halides, borides, and organometallic compounds.
Chemical Reaction of Samarium
- The Reaction of Samarium With Water
Under typical conditions, the reaction between samarium and oxygen (O2) takes place at a sluggish rate, resulting in the development of tarnish on the surface. Samarium exhibits a high propensity for combustion, resulting in the formation of Sm (III) oxide, commonly known as samarium (III) oxide or Sm2O3.
4 Sm (s) + 3 O2 (g) [when heated Δ] → 2 Sm2O3 (s)
- The Reaction of Samarium With water
The reaction between samarium and water behaves at a slow rate when subjected to cold water, while it proceeds rapidly when in contact with hot water. This reaction results in the formation of samarium hydroxide (Sm(OH)3) and the liberation of hydrogen gas (H2).
2 Sm (s) + 6 H2O (l) → 2 Sm(OH)3 (aq) + 3 H2 (g)
- The Reaction of Samarium With Halogens
The element samarium exhibits a propensity to engage in chemical reactions with various halogens, resulting in the formation of samarium (III) halides.
The chemical reaction between samarium metal and fluorine gas (F2), results in the formation of samarium (III) fluoride, denoted as SmF3.
2 Sm (s) + 3 F2 (g) → SmF3 (s) [white]
The chemical reaction between samarium metal and chlorine gas (Cl2), results in the formation of samarium (III) chloride, denoted as SmCl3.
2 Sm (s) + 3 Cl2 (g) → SmCl3 (s) [yellow]
The chemical reaction between samarium metal and bromine (Br2), results in the formation of samarium (III) bromide, denoted as SmBr3.
2 Sm (s) + 3 Br2 (g) → SmBr3 (s) [yellow]
The chemical reaction between samarium metal and iodine represented as (I2), results in the formation of samarium (III) iodide, denoted as SmI3.
2 Sm (s) + 3 I2 (g) → SmI3 (s) [orange]
- The Reaction of Samarium With Acid
Samarium is highly soluble in dilute sulfuric acid, forming a yellow solution with Sm (III) ions and releasing hydrogen gas (H2).
2 Sm (s) + 3 H2SO4 (aq) → 2 Sm3+ (aq) + 3 SO42− (aq) + 3 H2 (g)
Uses of Samarium
Numerous significant applications exist for samarium and its compounds some of which are discussed here:
Used In Magnets
The primary application of samarium lies in the production of samarium-cobalt alloy magnets, which find utility in various devices such as headphones, small motors, and pickups specifically designed for electric guitars. The magnets exhibit significant resistance to demagnetization. The ferromagnetic properties of the material are maintained at temperatures as high as 700°C. The utilization of SmCo magnets in precision-guided weapons is attributed to their capacity to function effectively under elevated temperatures.
Used As Catalyst
Samarium compounds have been found to serve as valuable catalysts in a diverse range of processes, including the decomposition of plastics, dechlorination of pollutants, Friedel-Crafts reactions, de-sulfonylation reactions, annulations, Barbier reactions, and more. Samarium oxide, also known as Samaria, serves as a catalyst in the processes of ethanol dehydration and dehydrogenation.
Used In Optics
The inclusion of samarium in glass can be employed to achieve specific optical characteristics or impart coloration. Additionally, it finds application in the production of lasers for specific purposes. A laser is an apparatus utilized to generate highly intense illumination characterized by a singular wavelength. The color emitted by a laser is contingent upon the constituent elements it incorporates.
Used In Nuclear Reactors
Samarium is employed as a nuclear reactor absorber in various applications. The process of radioactive decay involves the emission of subatomic particles, such as neutrons. The detrimental effects of radiation are attributed to its ability to cause damage to intricate chemical structures, such as DNA, within the human body. Samarium has the capability to effectively absorb neutrons emitted by nuclear reactors. The utilization of samarium-149 in nuclear reactor control rods is attributed to its notable neutron capture cross-section.
Used In Ceramics
Samarium oxides are commonly incorporated into a range of ceramic and glass materials in order to enhance their ability to absorb infrared radiation.
Health Effects of Samarium
Samarium lacks a clearly identifiable biological role; nevertheless, it has been observed to induce a stimulatory impact on metabolic processes. The consumption of soluble samarium salts may lead to moderate toxicity, whereas contact with samarium has been observed to induce discomfort of the skin and eyes, thereby presenting potential health risks.
Environmental Effects of Samarium
Samarium does not pose any discernible threat to the well-being of plants or animals. To date, there is a lack of documented cases reporting samarium poisoning in plant or animal species.
References
- https://www.rsc.org/periodic-table/element/62/samarium
- https://byjus.com/chemistry/samarium/
- https://www.britannica.com/science/samarium
- https://www.chemicool.com/elements/samarium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Samarium
- https://www.chemistrylearner.com/samarium.html
- https://education.jlab.org/itselemental/ele062.html
- https://www.thoughtco.com/samarium-facts-4136761
- https://www.livescience.com/38162-samarium.html
