Spectrophotometers are used to ascertain the wavelength distribution of light through the measurement of the percentage of reflectance from an object, as well as the identification of the color of the sample. Among two different kinds of beam spectrophotometer (double and single beam spectrophotometer), a single beam spectrophotometer has only one beam of light and is used to determine color by measuring the intensity of the light sources before versus after a test sample is inserted. The spectrophotometer finds utility in various applications, including the quantification of analyte concentration in solution through the measurement of absorbance at a specific wavelength and the application of the Beer-Lambert Law, as well as in kinetic assays.
Single beam spectrophotometers operate by transmitting a solitary beam of light through the sample holder. To standardize the instrument, a reference is inserted into the sample holder. The obtained value is then utilized to eliminate the impact of the solvent and the cell from subsequent sample measurements by subtraction. Single-beam instruments offer several benefits such as a wide dynamic range, uncomplicated optics, minimal moving components, and a condensed structure. Spectrophotometers are utilized in various applications, including the determination of analyte concentration in solution through absorbance measurement at a specific wavelength and the application of the Beer-Lambert Law, as well as kinetic assays.

UV-Vis spectrophotometers with a single beam configuration are capable of measuring within the wavelength range of 190 to 750 nm, with some models extending up to 1100 nm. The ultraviolet (UV) region, which encompasses wavelengths shorter than 340 nm, is a valuable tool for quantifying nucleic acids, purified proteins, and various other organic molecules. The selection of a suitable spectrophotometer is contingent upon the nature of the sample and the intended use. It is recommended to search for a suitable range of sample volumes, efficient sample changing capability, a display that is easily legible, and a unit that is simple to maintain and clean.
Interesting Science Videos
What is a single beam spectrophotometer?
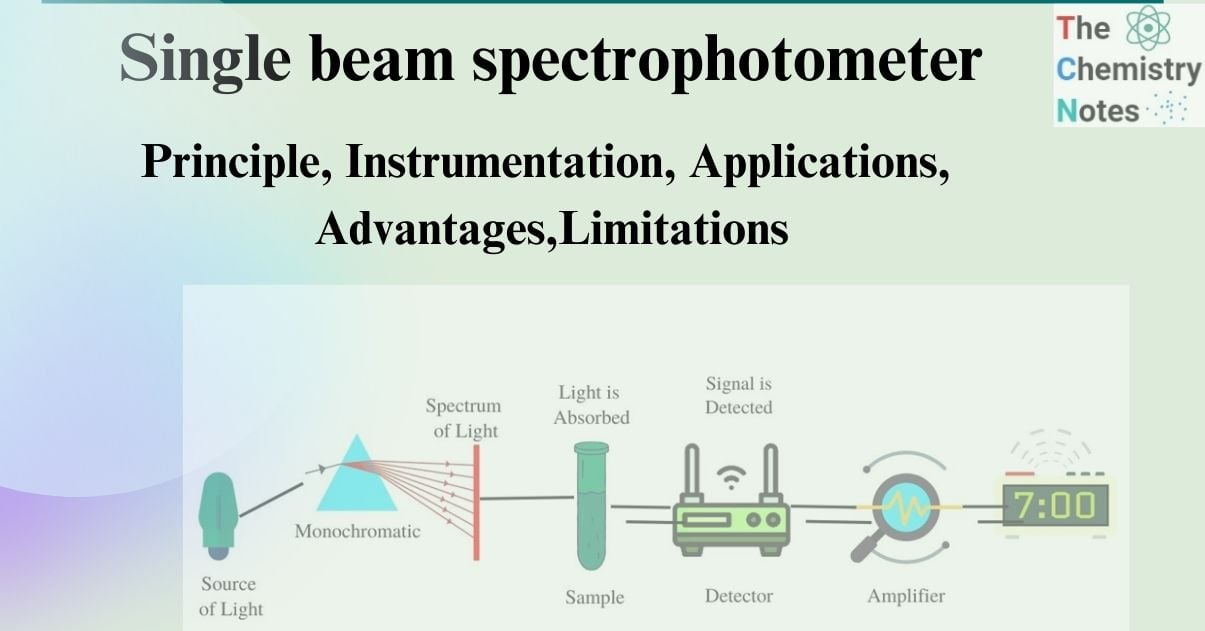
The single beam spectrophotometer is an analytical device wherein all light waves originating from the light source traverse through the sample. Consequently, the measurements are obtained by quantifying the light’s intensity prior to and subsequent to its transmission through the specimen. Single beam spectrophotometers are comparatively more compact and optically less complex than double-beam spectrophotometers.
In short, the color of an object can be determined using a single beam spectrophotometer by comparing the intensity of the light sources before and after a test sample is added. This light source is modulated, which means that its on and off states are changed, so that the light that comes from it can be distinguished from the light that comes from the flame. A single light beam is used to measure the sample by passing it through the reference, and a single beam spectrophotometer measures the intensity of the light that is reflected back from the reference.
Working principle of single beam spectrophotometer
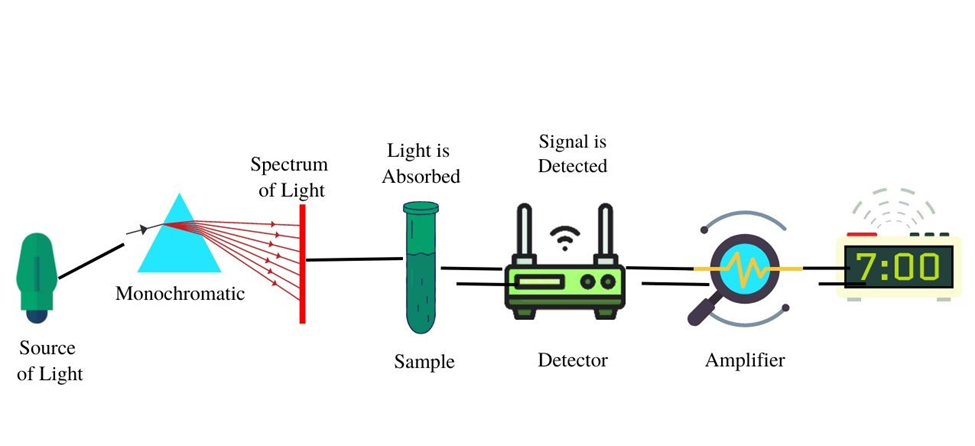
Figure: Single beam spectrophotometer (Source: https://www.scientificsarkar.com/single-beam-spectrophotometer/)
One type of spectrophotometer, known as a single-beam spectrophotometer, is an analytical instrument that lets all of the light waves that are emitted by the light source pass through the sample.
- Measurements are taken at various points throughout the process since the light’s intensity shifts as it passes through the sample.
- First, a spectrophotometer needs to be calibrated, which is done with the help of standard solutions that include a solute at a known concentration in the test solution. In order to accomplish this, the spectrophotometer’s Cuvette container is loaded with the standard solutions, and the Cuvettes themselves are then filled.
- A single light beam from a light source is used by single-beam spectrophotometers to illuminate both the sample and the reference sites. The monochromator is responsible for selecting the light wavelength that will be utilized in this particular instance.
- In the same ultraviolet spectrum, a single-beam spectrophotometer can accurately quantify both nucleic acid and purified protein samples. The amount of radioactive analyte that is present in a sample is determined by this process. The number of light beams that are absorbed by a particular component within the specimen can be counted to determine how this is performed.
- In this scenario, the Beer-Lambert Law provides an applicable tool for determining the size of the light beam. According to this law, the concentration of an analyte in the samples has a direct relationship with the absorbance factor in the samples.
Instrumentation of Single beam spectrophotometer
The single beam spectrophotometer comprises several crucial components, including:
- Light source: The spectrophotometer employs three distinct light sources to generate light of varying wavelengths. A tungsten lamp is the predominant light source utilized in spectrophotometry for the visible spectrum. The hydrogen lamp and the deuterium lamp are frequently utilized sources of Ultraviolet radiation. The Nernst filament or globar is widely regarded as the most optimal source of infrared (IR) radiation.
- Monochromator: The optical apparatus comprises an entrance slit and a dispersive element, which may take the form of a prism or a diffraction grating. In order to isolate a specific wavelength, either a prism or a diffraction grating is employed to disperse the light emanating from the light source.
- Sample holder: In order to store the colored solutions, either test tubes or cuvettes are utilized. At a wavelength visible to the human eye, they are composed of glass.
- Photodetector system: An electric current is produced whenever the light is allowed to fall on the detection system; this electric current then reflects the galvanometer measurement.
- Measuring device: The galvanometer, which is the measuring device, receives the current that is produced by the detector. The measurement on the meter is directly proportional to the amount of light that is there.
Figure: Instrumentation of single beam spectrophotometer
Procedure for single beam spectrophotometer
- Calibration: When utilizing a spectrophotometer, it is necessary to first calibrate the instrument. This is accomplished by the utilization of standard solutions containing a known concentration of the solute whose concentration is to be determined in the test solution. In order to accomplish this, the cuvettes are given their standard solution, and then they are placed in the holder for the cuvettes on the spectrophotometer, which is an instrument very similar to the colorimeter.
- A path towards monochromator: A ray of light with a particular wavelength that is tailored to the analysis is aimed at the solution. This ray of light is directed towards the solution. A ray of light will travel via a diffraction grating, a prism, and a succession of mirrors before finally arriving at the solution. The prism is what separates the beam of light into its component wavelengths, while the diffraction grating is what allows the needed wavelength to travel through it and make its way to the cuvette, which may contain either the standard or the test solutions. These mirrors are employed for the navigation of the light within the spectrophotometer. It performs an analysis on the reflected light and then makes a comparison to a previously established benchmark solution.
- Detecting system: Upon reaching the Cuvette, monochromatic light undergoes reflection, absorption, and transmission processes. Specifically, a portion of the light is reflected, while another portion is absorbed by the solution. The remaining light that passes through the solution is then detected by the photodetector system. The photodetector system quantifies the magnitude of transmitted light and transforms it into electrical signals, which are subsequently transmitted to the galvanometer.
- Current measurement: The galvanometer is utilized for the measurement of electrical signals and presents the results in a digital format. The absorbance or optical density of the analyzed solution is a digital representation of its electrical signals.
- Concentration of solution: The degree of light absorption by a solution is directly proportional to its absorption capacity. When the absorption capacity of the solution is high, a greater amount of light is absorbed by the solution. Conversely, when the absorption capacity of the solution is low, a greater amount of light is transmitted through the solution. This phenomenon has a direct impact on the galvanometer reading, which is indicative of the solute concentration in the solution. The concentration of a solution can be readily ascertained by inputting all the pertinent values into the equation of Beer-Lambert law.
Difference between single and double beam spectrophotometer
Parameters that differentiate single- and double-beam spectrophotometers are as follows:
1. Definition
- An analytical instrument known as a single light beam spectrophotometer is described as one that observes each individual light ray that originates from the light source as it travels through the specimen. Therefore, in this case, the measurements consist of taking the intensity of the light both before and after it has passed through the biological samples. The nature of the single-beam spectrophotometer is such that it is more compact than any double beam spectrophotometer, and it also has a simpler optical design. In spite of all of these factors, they are difficult to reproduce. In addition to that, these optical instruments are more reasonably priced.
- In most cases, a double beam spectrophotometer is the instrument of choice when it comes to determining whether analytes in a sample transmit or reflect light. The samples that are employed in this instance are either solids that are transparent or opaque, such as polished glass, or gases that are capable of functioning as solvents. It has been discovered that many different kinds of biochemicals have colors, which causes them to be able to take in visible light or ultraviolet light. Within the spectrophotometer, colorimetric processes can be used to measure the amount of light that has been transmitted. Even the colorless biochemicals that are used in this procedure have the potential to be transformed into colourful compounds. It is appropriate for use in chromogenic color-forming processes, which can then lead to the formation of compounds that are appropriate for colorimetric analysis using a single or double beam spectrophotometer.
2. Number of light beams
In a spectrophotometer with a double-beam design, the beam coming from the light source is separated into two distinct components. During the time that you are using the single beam spectrophotometer, there is only one beam that is produced by the light source. The double beam spectrophotometer uses two separate beams of light, each of which is directed at a different section of the instrument to illuminate it. The first section shines light on the benchmark standard, and the subsequent section shines light on all of the samples taken together. Before entering a single monochromator that is attached to the spectrophotometer, the light beams might be recombined. In certain applications of spectrophotometry, the use of two monochromators is necessary. In this particular instance, the dividing of the light beam can typically be accomplished in either of these two ways:
- The first one is done statistically, and it involves a mirror that only transmits some of the light or another device that is quite similar to it together with the actual instrument.
- by lowering the intensity of the light beams being measured by the double beam spectrophotometers by the utilization of movable optical and mechanical elements.
Because of their impressive feature set, double beam spectrophotometers have gained significant market share. When using a single beam spectrophotometer, the light beam that comes from the light source is focused in one direction, and its job is to illuminate both the specimen and the reference points. The wavelength of light that is utilized here is decided by the monochromator.
3. Nature of light beams:
- A single beam spectrophotometer employs a light beam that is of the single nonsplit kind, as its name suggests. Consequently, the light beams cannot be separated in any way while the spectrophotometry procedure is being carried out.
- In the case of spectrophotometers that use a double beam, the light beam that is used is one that is split in two. Before the light beams go through the biological samples, they are first divided into two portions as a result of the splitting that takes place.
4. Measurement:
- A single beam of light is used as the source for all of the measurements that are collected by a single-beam spectrophotometer. However, due to the fact that only a single laser beam is utilized throughout the measurement process, the results of these measurements are less reliable. The single beam spectrophotometers were developed as a result of this.
- On the other hand, the readings that are produced by the double beam spectrophotometer are extremely reproducible. Due to the fact that it applies electronic and mechanical effects to biological samples while simultaneously positioning reference beams in the same space. Therefore, as a result of this, the double beam spectrophotometer is utilized significantly more frequently than the single beam spectrophotometers. In this instance, the beam of light has an effect on references as well as samples that are located in the same location.
5. Measured parameters:
- In the context of a spectrophotometer with a single beam, the instrument determines the amount of an analyte that is present in a sample. In order to determine this, the amount of light that is absorbed by the analyte in question in the specimen is measured. When it comes to the measurement of the light beams, the Beer-Lambert Law is put into action here. The concentration of an analyte is said to have a relationship that is directly proportional to the absorbance factor, as stated by this law.
- The absorbance of the biological sample can be determined with the help of a double beam spectrophotometer. The rate of absorption that occurs within the instrument can be measured using the reference beam of light. At this point, the sample can be contrasted with the reference point that was utilized. Therefore, the ratio between the sample beams utilized in spectrophotometry is considered to constitute the definition of absorption. After traveling through both the sample and a reference beam of light, the light beams are next subjected to measurement. A monochromator is incorporated into a double beam spectrophotometer. This component separates the desired wavelengths from a light beam. Before entering the monochromator, these reference light beams and sample light beams unite into a single beam of light. As a result, the measurement is carried out.
An optical instrument known as a spectrophotometer analyzes the analytes in a sample by examining how well the analytes can absorb light. The single beam spectrophotometer and the double beam spectrophotometer are the two primary varieties of spectrophotometers that may be purchased nowadays. The primary distinction between a single beam and a double beam spectrophotometer is that, in a single-beam spectrophotometer, all of the light rays are directed through the biological sample. In a double-beam spectrophotometer, only some of the light rays are directed through the sample. While in a device called a double beam spectrophotometer, the light beam is divided into two pieces, and only one of those parts is allowed to pass through the sample. Only one of the sample’s absorbances or the reference blank’s absorbance will be counted at a time.
Advantages of single beam spectrophotometer
- Enhanced productivity
- The ability to detect with a high level of sensitivity.
- Less expensive
- Simple to use
- Single beam spectrophotometers have the capability of measuring a broad spectrum of wavelengths, which enables them to be utilized in a wide variety of settings and contexts.
Limitations of single beam spectrophotometer
- The method fails to consider potential errors such as voltage fluctuations that may impact the results.
- The measurement of absorbance is limited to either the sample or the reference blank at a given moment.
Applications of single beam spectrophotometer
Single beam spectrophotometers are analytical instruments that find widespread application across many disciplines, such as the sciences of chemistry, biology, and environmental science. The following are some examples of common applications:
- Analyzing the concentration of chemical compounds in solutions,
- Studying the purity of drugs,
- Measuring the pH of a sample,
- Analyzing the composition of food and beverages, such as wine and beer.
Summary of Single beam spectrophotometer
- A sort of analytical device known as a single-beam spectrophotometer is utilized for the purpose of determining how much light a given sample has taken in. A light source, a monochromator to pick the wavelength of light that is needed, a sample holder, and a detector are the components that make up this apparatus. The detector measures the intensity of the light after it has passed through the sample.
- A spectrophotometer then measures the amount of light that is absorbed by the sample at a particular wavelength after the sample has been placed in the sample holder. The result is often conveyed as the sample’s absorbance, which is a measurement of the extent to which the sample absorbs light of a specific wavelength.
- In this process, a single laser beam is utilized that has a wavelength that ranges from 325 nm to 1000 nm. Because the light can only travel in one direction, the readings for the test solution and the blank are taken in the same direction. It works wonderfully for determining the amount of light that is absorbed or transmitted at a specific wavelength. It is not for scanning the spectrum across all bands. Because of the increasing requirements for both the light source and the detector stability.
- In addition to their usage in chemical analysis and medicinal research, single-beam spectrophotometers are also frequently put to use in the field of environmental monitoring.
References
- https://www.smacgigworld.com/blog/difference-single-beam-spectrophotometer-and-double-beam-uv-vis-spectrophotometer.php
- https://www.slideshare.net/MuhammadKashifHanif1/single-beam-spectrophotometer
- https://www.scientificsarkar.com/single-beam-spectrophotometer/
- https://www.studocu.com/row/document/government-college-women-university-faisalabad/inorganic-chemistry/single-and-double-beam-spectrophotometre/15742585
