A heterogeneous class of substances known as solvents can be used to dilute, dissolve, or spread other molecules. The chemical makeup and physical characteristics of both the solvent and the solute affect a solvent’s capacity to dissolve another molecule. The major part of a solution is composed of solvent while the minor part is composed of solute. The solvent is the substance or liquid in which other materials dissolve to form a solution.
Interesting Science Videos
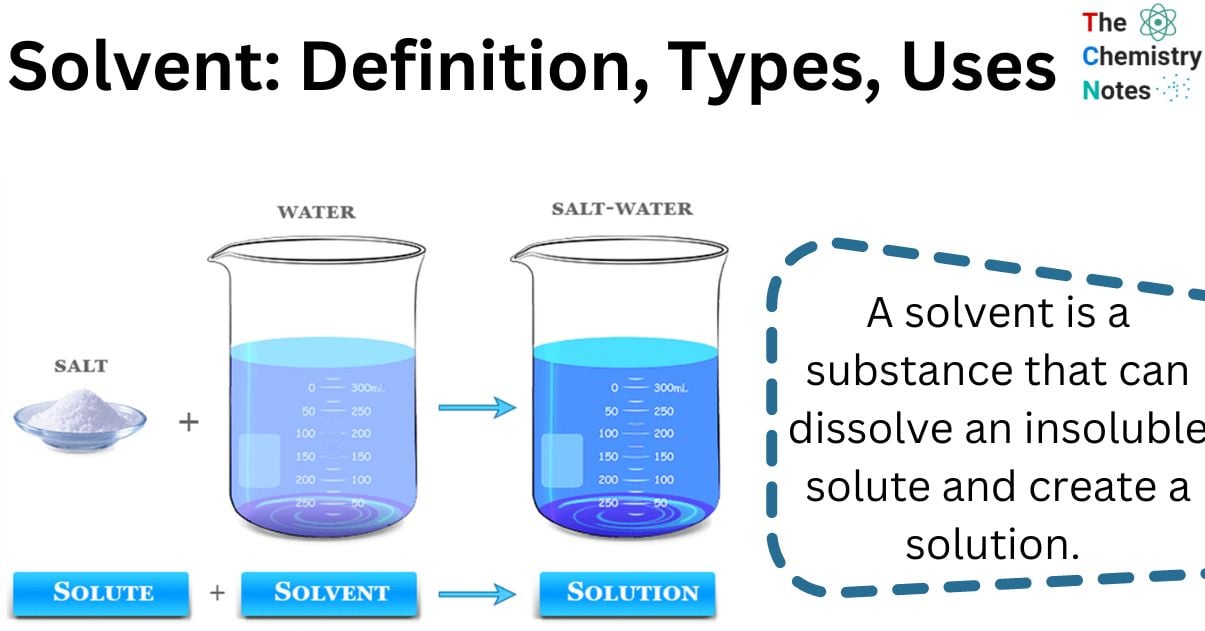
What is a Solvent?
A solvent is a substance that can dissolve an insoluble solute and create a solution. Solvents are often liquids, although they can also be solids, gases, or supercritical fluids.
The amount of solute that dissolves in a given volume of solvent changes depending on the temperature. Dry cleaning, paint thinners, nail polish removers, glue solvents, spot removers, detergents, and scents are a few applications of solvents. Polar molecules can be dissolved in water. Water dissolves practically all solutes, making it the most used solvent.
Proteins and ions found inside a live cell dissolve in water as well. For chemical synthesis and purification operations, solvents are used in a variety of ways in the chemical, pharmaceutical, oil, and gas industries.
Types of Solvent
Polar and nonpolar molecules are the two main categories of molecules. Electrical charges are separated on the opposite surfaces of polar molecules. Nonpolar molecules do not have a static charge, yet their charge can change. As explained below, both kinds of molecules can function as solvents.
Polar Solvent
Polar solvents function by interacting with both the solute and the positive and negative ends of each atom. Through the electrical charges on various solute molecule portions, a polar solvent dissolves a solute. Ionic substances, such as salt, can be dissolved by polar liquids by drawing on the molecules with opposing charges. The negative ions are drawn to the positive sides of other solvent molecules. The ions are then equally dispersed throughout the solvent in this manner.
Nonpolar Solvent
Nonpolar solvents function in the same manner as polar solvents do. Nonpolar compounds that function as solvents are frequently spontaneous dipoles, meaning they occasionally create opposing electrical charges between bonds. These transient electrical dipoles lead to the formation of dipoles in surrounding solvent molecules. Other nonpolar molecules can dissolve as a result of these short interactions.
Polar compounds, however, frequently interact more strongly with one another than they do with the transient dipoles of nonpolar molecules. Because of this, polar and nonpolar solvents, such as water and oil, cannot be combined. Fats, oils, and greases are non-polar solutes that do not dissolve in water. They combine to generate an emulsion when combined.
Examples: n-hexane; benzene; toluene; diethyl ether; chloroform; 1,4-dioxane
Aprotic Solvents
Aprotic solvents do not release protons; instead, they can function as either a simple solvent, where the dielectric constant indicates considerable polarity or as an aprotic basic, a proton acceptor. Aprotic solvents are polar liquid molecules devoid of hydrogen atoms that can be broken apart. These solvents don’t include any chemical entities like O-H or N-H bonds. As a result, hydroxyl (-OH) and amine (-NH2) groups aren’t present in aprotic solvents and can’t establish hydrogen bonds.
Protic and aprotic solvents both have the capacity to dissolve ions. These solvents don’t contain much acidic hydrogen, therefore there isn’t a significant release of hydrogen ions. Dielectric constant values for polar aprotic solvents are modest or moderate. These solvents have a moderate degree of polarity.
Examples: Ethyl acetate; tetrahydrofuran; dichloromethane; acetone; acetonitrile; dimethylformamide; dimethyl sulfoxide.
Protic Solvents
A protic solvent is composed of molecules that could act as hydrogen-bond donors. Alcohol, water, and carboxylic acids are some examples of protic solvents.
Polar protic solvents are compounds with the general formula ROH. The polarity of polar protic solvents is caused by the dipole of the O-H bond.
Examples: Acetic acid; n-butanol, n-propanol, ethanol, formic acid; isopropanol
Solvent Examples
They can also be classified based on their chemical composition.
- Inorganic solvents: water and ammonia
- organic solvents contain both carbon and oxygen: alcohols and glycol ethers.
- Hydrocarbon solvents only contain carbon and hydrogen: gasoline, benzene, toluene, and hexane.
- Halogenated solvents contain halogens such as chlorine (Cl), fluorine (F), bromine (Br), or iodine (I) in their composition (CFCs): carbon tetrachloride, chloroform, and chlorofluorocarbons.
Uses of Solvent
They have broad applications in normal household works to industrial works. They are used in a wide range for dissolving oil, paints, mixing pigments, glues, and, resins and all these have their particular uses. They are used in cleaning automotive parts, electronics, painting, mixing chemicals, etc. They have a wide range of applications in the chemical, pharmaceutical, oil, and gas industries, including chemical synthesis and purification.
The following applications all use solvents in the manufacturing process;
- Pharmaceuticals
- Coatings and Paints
- Inks for printing
- Personal Care and Cosmetics
- Food and Drink
- Degreasing and cleaning
Solvents and Use
- Tetrachloroethylene: Dry cleaning;
- Toluene, turpentine: paint thinners;
- Acetone, methyl acetate, ethyl acetate: nail polish removers and glue solvents
- Hexane, petrol ether: spot removers;
- ethanol: Perfumes;
- citrus terpenes: detergents;
Health Effects of Solvents Exposure
Some of the general health risks associated with solvent exposure are listed below:
Acute exposure
Many solvents, when inhaled in large quantities, can cause a sudden loss of consciousness. Solvents such as diethyl ether and chloroform have long been used in medicine as anesthetics, sedatives, and hypnotics. Ethanol (grain alcohol) is a popular and highly abused psychoactive drug. Diethyl ether, chloroform, and many other , such as those found in gasoline or glues, are abused recreationally in glue sniffing, often with negative long-term health consequences such as neurotoxicity or cancer.
Long-term exposure
Some solvents, such as chloroform and benzene, a common ingredient in gasoline, are known carcinogens, while the World Health Organization considers many others to be likely carcinogens. They can harm internal organs such as the liver, kidneys, nervous system, and brain. The cumulative effects of long-term or repeated exposure to solvents are called chronic solvent-induced encephalopathy (CSE).
Chronic exposure to organic solvents in the workplace can result in a variety of negative neuropsychiatric effects.
Contamination of the environment
Spills or leaks of solvents that reach the underlying soil are a major source of health effects. Because they can easily migrate long distances, widespread soil contamination is not uncommon; this is especially dangerous if aquifers are affected.
Reference
- Kosower, E.M. (1969) “An introduction to Physical Organic Chemistry” Wiley: New York, p. 293
- Lowery TH, Richardson KS (1987). Mechanism and Theory in Organic Chemistry (3rd ed.). Harper Collins Publishers. ISBN 978-0-06-364044-3.
- Tinoco, I.; Sauer, K.; Wang, J.C. (2002). Physical Chemistry. Prentice Hall. ISBN 978-0-13-026607-1.
- https://www.solvents.org.uk/solvents-and-you/common-uses-of-solvents/
- https://www.toppr.com/guides/chemistry/solutions/solvent/
- Hung T, Dewitt CR, Martz W, Schreiber W, Holmes DT (July 2010). “Fomepizole fails to prevent progression of acidosis in 2-butoxyethanol and ethanol coingestion”. Clinical Toxicology.
