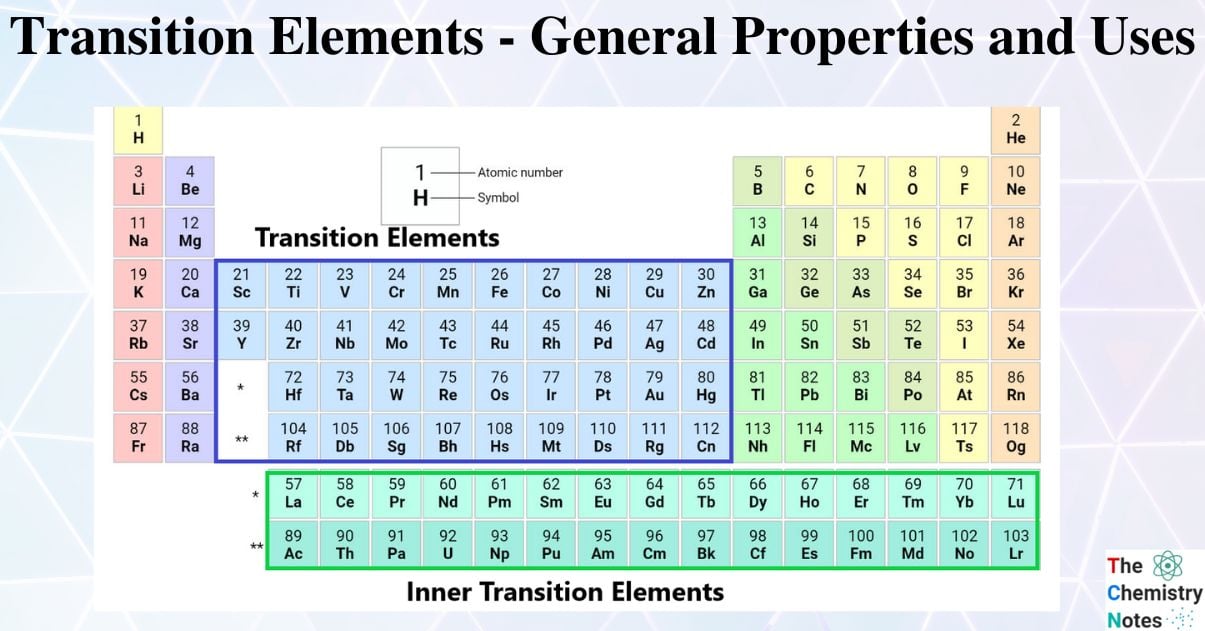
The transition metals, which are located in the middle of the periodic table, make up the largest group of elements there. Additionally, the lanthanides and actinides, two rows of elements below the main body of the periodic table, are particular subsets of these metals.
Interesting Science Videos
What is Transition elements?
Transition elements on the periodic table are the d-block chemical elements lying between p-and s-block elements. These elements have a partially full (n-1)d orbital in either their atomic state or any of their typical oxidation numbers or states.
As a result of their atoms’ electrons filling the d subshell or d sublevel orbital, these elements are known as “transition metals.” Thus, the d-block elements and transition metals are both used interchangeably.
The inner transition elements, which are listed below the periodic table, contain 14 elements after Lanthanum, namely Cerium (58) to Lutetium (71), and 14 elements after Actinium, namely Thorium (90) to Lawrencium (103) are termed as “f-block elements“
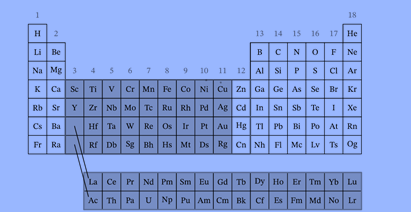
According to the IUPAC definition, the elements in the periodic table above marked in darker color are transition elements. The f-block elements, also transition elements and referred to as the inner transition metals, are the elements in the lanthanide and actinide series.
Classification of Transition Elements
Elements of the d- Block are divided into four series:
In the case of the d-block element, there are three complete rows—first, second, and third transition series—involving the filling of the 3d, 4d, and 5d orbitals, respectively, and one incomplete row—fourth row.
First transition series (3d-series): Scandium (21), Zinc (30), and other elements make up the first transition series. According to the periodic table, these elements belong to the fourth period.
Second transition series (4d-series): Yttrium (39), Cadmium (48), and other elements make up the second transition series. These elements are found in the fifth period of the periodic table.
Third transition series (5-d series): Lanthanum (57), Hafnium (72), and Mercury (80) make up the third transition series (5-d series). They are in the sixth period of the periodic table.
Fourth transition series (6-d series): Actinium (89) and the remaining elements from Rutherfordium (104) to Copernicium (112) make up the fourth transition series (6-d series). They are in the still-unfinished and located in seventh period.
Electronic Configuration
All d-block elements, also known as transitional elements, are divided into four series: 3d, 4d, 5d, and 6d. Ten chemical elements make up each series in the 3d, 4d, 5d, and 6d.
The general valence shell electronic configuration is (n-1)d1 to 10 ns0, 1, 2
Electronic configuration is actually thought to be (n-1) d1-9 ns 1-2, where (n-1) is the penultimate shell, due to their incomplete inner d- orbitals (second outermost shell).
Due to the fact that their inner d-orbitals are fully filled, group IB (11) elements like Cu, Ag, and Au, do not match the electronic configuration of transition elements. As a result, these components are recognized as belonging to the standard transition elements.
The table below displays the first d-series transition metals’ complete electronic configuration.
| Transition Elements | Symbol | Atomic Number | Electronic Configuration |
| Scandium | Sc | 21 | [Ar] 3d1 4s2 |
| Titanium | Ti | 22 | [Ar] 3d2 4s2 |
| Vanadium | V | 23 | [Ar] 3d3 4s2 |
| Chromium | Cr | 24 | [Ar] 3d5 4s1 |
| Manganese | Mn | 25 | [Ar] 3d5 4s2 |
| Iron | Fe | 26 | [Ar] 3d6 4s2 |
| Cobalt | Co | 27 | [Ar] 3d7 4s2 |
| Nickel | Ni | 28 | [Ar] 3d8 4s2 |
| Copper | Cu | 29 | [Ar] 3d10 4s1 |
| Zinc | Zn | 30 | [Ar] 3d10 4s2 |
| Yttrium | Y | 39 | [Kr] 4d1 5s2 |
| Zerconium | Zr | 40 | [Kr] 4d2 5s2 |
| Niobium | Nb | 41 | [Kr] 4d4 5s1 |
| Molybdenum | Mo | 42 | [Kr] 4d5 5s1 |
| Technetium | Tc | 43 | [Kr] 4d5 5s2 |
| Ruthenium | Ru | 44 | [Kr] 4d7 5s1 |
| Rhodium | Rh | 45 | [Kr] 4d8 5s1 |
| Palladium | Pd | 46 | [Kr] 4d10 |
| Silver | Ag | 47 | [Kr] 4d10 5s1 |
| Cadmium | Cd | 48 | [Kr] 4d10 5s2 |
Lanthanide and Actinide Series
| Transition element | Symbol | Atomic number | Electronic configuration |
| Lanthanum | La | 57 | [Xe] 4f0 5d1 6s2 |
| Hafium | Hf | 72 | [Xe] 4f14 5d2 6s2 |
| Tantalum | Ta | 73 | [Xe] 4f14 5d3 6s2 |
| Tungsten | W | 74 | [Xe] 4f14 5d4 6s2 |
| Rhenium | Re | 75 | [Xe] 4f14 5d5 6s2 |
| Osmium | Os | 76 | [Xe] 4f14 5d6 6s2 |
| Iridium | Ir | 77 | [Xe] 4f14 5d7 6s2 |
| Platinum | Pt | 78 | [Xe] 4f14 5d9 6s1 |
| Gold | Au | 79 | [Xe] 4f14 5d10 6s1 |
| Mercury | Hg | 80 | [Xe] 4f14 5d10 6s2 |
| Actinium | Ac | 89 | [Rn] 5f0 6d1 7s2 |
| Rutherfordium | Rf | 104 | [Rn] 5f14 6d2 7s2 |
| Dubnium | Db | 105 | [Rn] 5f14 6d3 7s2 |
| Seaborgium | Sg | 106 | [Rn] 5f14 6d4 7s2 |
| Bohrium | Bh | 107 | [Rn] 5f14 6d5 7s2 |
| Hassium | Hs | 108 | [Rn] 5f14 6d6 7s2 |
| Meitnerium | Mt | 109 | [Rn] 5f14 6d7 7s2 |
| Darmstadtium | Ds | 110 | [Rn] 5f14 6d8 7s2 |
| Roentgenium | Rg | 111 | [Rn] 5f14 6d9 7s2 |
| Copernicium | Cn | 112 | [Rn] 5f14 6d10 7s2 |
The idea of half-filled and completely-filled d-orbitals, which are comparatively more stable than other d-orbitals, is used to explain the abnormalities in the reported configurations of Cr, Cu, Mo, Pd, Ag, and Au.
24Cr= [Ar] 3d44s2 and 29Cu= [Ar] 3d94s2 are the predicted electronic configurations of Chromium and Copper respectively, whereas,
24Cr= [Ar] 3d54s1 and 29Cu= [Ar] 3d104s1 are the observed electronic configurations, where Cr and Cu are relatively more stable.
General Properties of Transition Elements
The 24 elements in issue have some notable similarities, including the fact that they are all metals, the majority of them are tough, strong, and lustrous, have high melting and boiling temperatures, and are effective heat- and electricity-conductors. Because the range of these attributes is wide, the statements are comparable to the generic properties of all the other elements.
- Transitional metals have a metallic appearance. However, gold and copper have colors that are not found in any other element on the periodic table. Most transition metals, like iron or silver, are grayish or white.
- The melting points of the transition metals are high as a whole. Mercury is an exception because it is a liquid at ambient temperature. These substances consequently have high boiling points.
- The energy difference between these elements’ potential oxidation states is quite small. As a result, the transition elements have a variety of oxidation states.
- Transition metals can produce colorful compounds and solutions because they can form colored complexes. To absorb particular light wavelengths, the complexes divide the d orbital into two energy sublevels
- Although reactive, the transition metals are not as reactive as the alkali metals group’s members.
- Since most transition metal ions and complexes have unpaired electrons in their (n-1) d-orbitals, they are paramagnetic, or attracted to the magnetic field.
- Transition metals form Interstitial compounds.
Application and Use of Transition metal
The use of transition metals is directly influenced by their physical and chemical characteristics. Because of their numerous applications, transition metals are immensely significant both socially and commercially.
- Steel alloys, which are commonly utilized in engineering, primarily consist of iron. Iron can also operate as a catalyst, most notably in the Haber-Bosch method for producing ammonia gas (NH3 ).
- Copper is mostly utilized in electrical wiring, but it is also the major element in bronze alloys.
- Because of its endurance, titanium is often utilized in alloys as well as dental implants. The substance titanium dioxide (TiO2) is utilized in sunscreen lotions, where it aids in shielding the skin from ultraviolet (UV) light’s damaging impacts.
- A crucial catalyst utilized in the contact method to create sulfuric acid (H2SO4 (l)) is the substance vanadium pentoxide (V2O5).
- Chromium is frequently used in metal plating, such as on the motorbike engine components . Additionally, it is employed in the tanning of leather.
References
- Smith, D. (1990). Inorganic Substances: A Prelude to the Study of Descriptive Inorganic Chemistry (Cambridge Texts in Chemistry and Biochemistry). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511622922
- Lee, J D. Concise Inorganic Chemistry. London: Blackwell Science, 2006. Print.
- Cotton, F A, and F A. Cotton. Advanced Inorganic Chemistry. , 1999. Print.
- Mingos, D. M. P. Essential Trends in Inorganic Chemistry. Oxford University Press, 1998.
- https://www.britannica.com/science/transition-metal
- https://onlinesciencenotes.com/electronic-configuration-and-general-properties-of-d-block-elements-or-transition-elements/
- https://en.wikiversity.org/wiki/Chemistry_(A-Level)/ Transition_elements
- https://byjus.com/chemistry/transition-elements/
