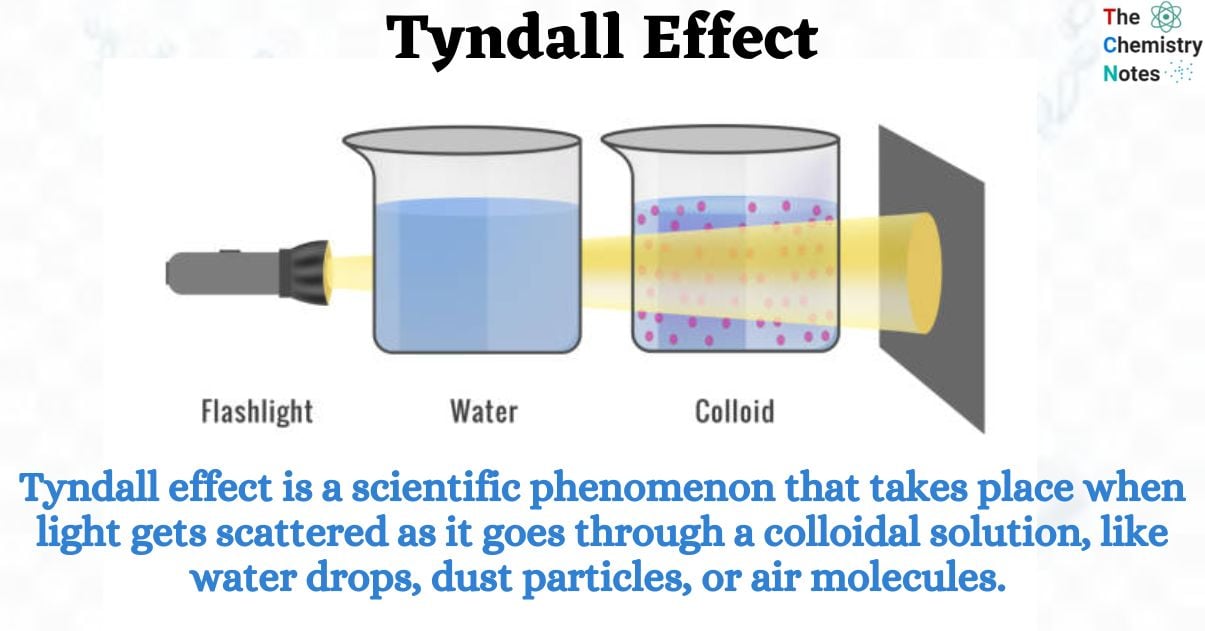
The Tyndall effect is a scientific phenomenon that takes place when light gets scattered as it goes through a colloidal solution, like water drops, dust particles, or air molecules.
The phenomenon mentioned in this statement is called the Tyndall effect. It was named after John Tyndall, an Irish physicist who presented evidence for this phenomenon in the year 1869. The term you mentioned is known as the Tyndall effect. When a beam of light travels through a substance that has small particles, such as colloids or fine particles, this phenomenon takes place. The light is scattered by these particles, which makes it visible. This also allows us to observe both the particles and the path of the light beam. An instance where dust particles in a room may become visible is when they come into contact with a beam of light that enters through a window.
The degree of scattering is determined by two factors: the frequency of the light and the density of the particles. The Tyndall effect is a scientific occurrence in which particles present in a substance disperse light, causing it to become visible. The Tyndall effect causes blue light to scatter more intensely than red light, just like Rayleigh scattering. One possible viewpoint is that light with longer wavelengths possesses the capability to traverse through a medium, whereas light with shorter wavelengths has a tendency to rebound or disperse.
Interesting Science Videos
What is Tyndall Effect
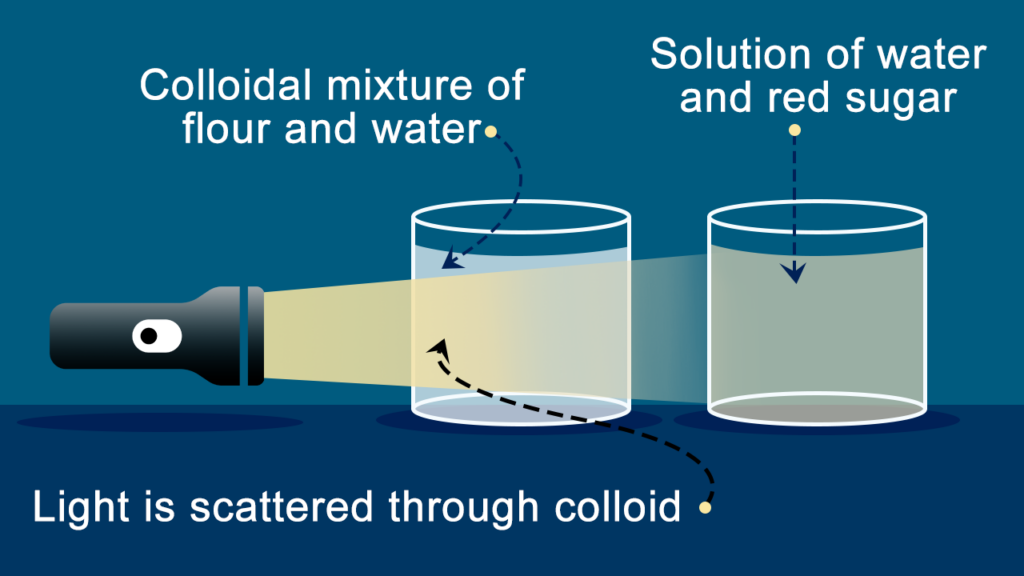
The Tyndall effect refers to the occurrence where particles present in a colloid disperse or scatter beams of light that are aimed towards them. All colloidal solutions and certain extremely fine suspensions demonstrate this effect. Hence, it can be utilized to confirm whether a provided solution is a colloid. The intensity of scattered light is influenced by both the density of the colloidal particles and the frequency of the incident light.. This is because colloidal solution particles match light beam wavelength. Therefore, the beam of light scatters and its route is apparent through the solution. This light scattering through colloidal solution is called Tyndall Effect.
Causes of Tyndall Effects
The Tyndall Effect occurs when the visible spectrum of light interacts with the particles present in a colloidal solution. When particles interact more with the light beam, the scattering of light also increases, which makes it more likely to observe the Tyndall effect. The Tyndall effect is a distinctive property exhibited by colloidal solutions, enabling a clear differentiation between real solutions and colloidal solutions.
- The visible spectrum of light includes wavelengths ranging from 400 nm – 700 nm. Within this spectrum, blue light is characterized by wavelengths between approximately 400 nm – 500 nm, while red light is situated within the range of 600 nm – 700 nm.
- The reason why the Tyndall effect is not observed in a true solution is because the particles that make up the solution are very small, usually less than 1 nm in size. This is smaller than the wavelength of visible light, which is why we can’t see the scattering of light in a true solution.
- If the size of the particles in a colloidal solution is similar to the wavelength of visible light, then the particles can scatter the light in different directions. This scattering makes it possible for us to see the path of the light beam.
Properties of Colloids
- A colloid is a mixture that contains particles with sizes between 1 – 100 nm.
- The mixture is considered heterogeneous because it contains particles that are denser than a solution, which results in the ability for light to pass through.
- The Tyndall Effect is a scientific phenomenon that happens when there are colloids present and a beam of light is passed through them.
- The visibility of the light beam’s path is caused by this effect. Colloids, such as smoke and sunlight, are mixtures that can be seen as examples.
The properties that differentiate colloids from true solutions and suspensions are as follows:
Heterogeneous Mixture
- Heterogeneous Mixture refers to a combination of substances that are not uniformly distributed throughout, resulting in visible differences in composition or properties within.
- As a heterogeneous mixture colloid is composed of two distinct phases:
- Dispersed Medium
- Dispersion Medium
Stability
- The particles of colloids exhibit perpetual motion. A limited number of colloid particles, characterized by greater sizes, exhibit a gradual sedimentation process over a specific time interval.
Filtration
- The colloidal solution contains particles that are sufficiently small to permeate regular filter sheets, yet they can be effectively retained by various types of membranes, including animal membranes, cellophane membranes, and ultrafilters.
Color
- The color of a colloidal solution is determined by the presence of colloidal particles within it.
- Large particles absorb light with longer wavelengths, resulting in the transmission of light with shorter wavelengths.
- The opposite is also true, where big particles transmit light with shorter wavelengths and absorb light with longer wavelengths.
Other Properties
- In addition to their colloidal nature, colloidal solutions exhibit various distinctive phenomena.
- Brownian motion: It is the random motion of particles suspended in a fluid.
- Electro-osmosis: It is a phenomenon that occurs in certain materials, such as porous solids or cap.
Examples of Tyndall Effects
The Tyndall effect exhibits numerous instances that can be noticed within our immediate surroundings upon attentive examination. The following are a few examples.
- Milk is classified as a colloid due to its composition, which includes tiny globules of fat and protein. When a beam of light interacts with a glass of milk, it undergoes a phenomenon known as scattering, resulting in the light being dispersed in various directions. This serves as an excellent illustration of the Tyndall effect.
- Opalescent glass displays a characteristic bluish tint when viewed from a lateral viewpoint. When light traverses the medium of glass, it engenders a perceptible optical phenomenon characterized by the appearance of an orange color.
- In a setting with limited visibility caused by fog, the utilization of a portable light source effectively illuminates the nearby surroundings, thus making it possible to perceive the path of the emitted light. The occurrence of light dispersion in this particular situation can be ascribed to the presence of water droplets within the fog.
Tyndall Effect and Blue Color of Eyes
The primary differentiation between irises exhibiting blue, brown, and black pigmentation is attributed to the varying concentrations of melanin within a specific layer.
- The layer present in a blue iris exhibits a comparatively diminished concentration of melanin in comparison to a black iris, resulting in its translucent nature.
- When light interacts with a translucent layer, it experiences scattering due to the Tyndall effect.
- The disparity in wavelength between blue and red light results in a more pronounced scattering phenomenon experienced by the former.
- The iris exhibits an additional layer that plays a crucial role in the assimilation of non-dispersed light.
- The irises obtain their characteristic blue coloration as a result of the abundance of blue light present in the scattered light.
Frequently Asked Questions (FAQ)
Does the Tyndall effect disappear by itself?
The Tyndall effect will persist as long as the dermal filler is present, although the intensity of the phenomenon may diminish as the particles of the filler degrade.
Does sodium chloride show Tyndall effect?
The Tyndall effect does not occur in sodium chloride due to the small size of its particles. The sodium chloride solution contains a high percentage of particles with a very small size. When the beam of light passes through the solution, it undergoes a certain effect or behavior. The presence of colloid particles in a solution causes partial obstruction of the beam, preventing it from passing through completely. The reason why the Tyndall effect is not observed in a solution is because of a specific factor.
Which color of light is scattered the most in the atmosphere?
The molecules of air in the atmosphere are smaller than the wavelength of visible light. You may already be aware that particles of a very small size tend to scatter only blue colors. They are better at scattering light with shorter wavelengths.
Why does the Tyndall effect not work on true solutions?
The Tyndall effect does not occur in true solutions because it is a phenomenon that is only observed in colloidal suspensions or mixtures, not in solutions.
This concept is based on the understanding that when there are large colloidal particles present in a solution, they cause light beams to scatter and separate. In a true solution, the particles are too small to scatter light, which is why the Tyndall effect is not observed.
Video on Tyndall Effect
References
- https://collegedunia.com/exams/tyndall-effect-science-articleid-401
- https://unacademy.com/content/neet-ug/study-material/chemistry/tyndall-effect-and-properties-of-colloids/
- https://www.geeksforgeeks.org/tyndall-effect/
- https://www.thoughtco.com/definition-of-tyndall-effect-605756
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Solutions_and_Mixtures/Colloid/Tyndall_Effect

