There are two main types of isomers: structural isomers and stereoisomers. Isomers are structures that share the same molecular formula and chemical composition but have distinct atomic arrangements in space.
Interesting Science Videos
Introduction to Isomer
Isomers are chemical compounds that have the same chemical formula but differ in their properties and atom configurations. Isomers are, therefore, compounds that exhibit isomerism. One of the most important properties of organic compounds is their ability to display isomerism. Isomerism is a feature of a material that occurs when two or more compounds with the same molecular formula but different physical and chemical properties exist.
Friedrich Wohler, a chemistry expert, synthesized cyanic acid in 1827 and was struck to find that the chemical structure of the generated substance was similar to that of fulminic acid, but the characteristics of this acid were considerably different. This revelation sent shockwaves across the scientific community since it was previously assumed that chemical compounds could only have diverse properties if they were made up of separate elements. Following different discoveries, this was recognized as a scientific phenomenon, and Jacob Berzelius termed it Isomerism.
The term “isomer” comes from the Greek words “isos” and “meros” both of which signify “equal parts.” In 1830, the Swedish scientist Jacob Berzelius came up with the name.
Types of Isomers
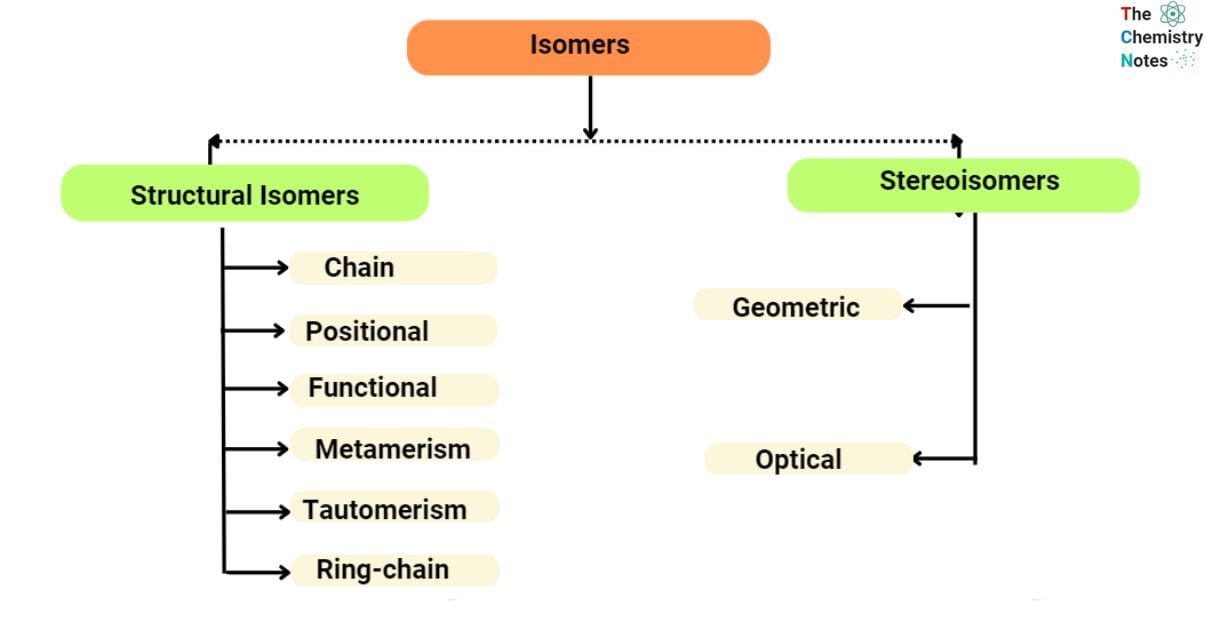
Isomerism is classified into two main kinds, each of which has several subgroups. Structural isomerism and stereoisomerism are the two most common forms.
Structural Isomerism
Even though the structural isomers have the same chemical formula, their atoms are arranged in radically different ways. These molecules have similar chemical formulas, but depending on how they are combined, they have various connectivity’s. Structural isomerism is also known as constitutional isomers. Since separate structural isomers may or may not include the same functional group, they are assigned distinct IUPAC nomenclature.
Several kinds of structural isomerism are discussed here:
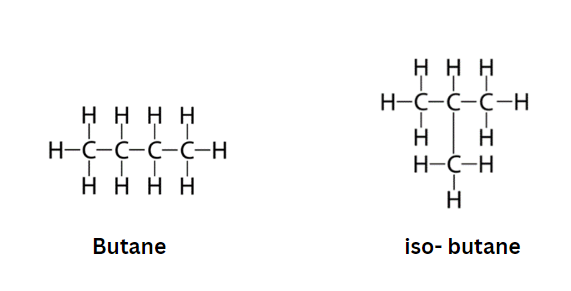
Chain Isomerism/Skeletal Isomerism
Chain isomerism does not exist in molecules containing up to three carbon atoms since they are a continuous chain with no possibility of branching. It is proved for molecules containing four or more carbon atoms.
- The configuration of the carbon chain differs for different isomers, which is known as chain isomerism or skeletal isomerism.
- The components of these isomers exhibit a variety of branch structures. The way the carbon is branched commonly varies amongst chain isomers.
- The most fundamental hydrocarbons, such as methane, ethane, and propane, don’t have any structural isomers.
- Butane (C4H10), a hydrocarbon containing four carbon atoms, is the smallest hydrocarbon that may display this isomerism. Isobutane, a branched chain isomer, and n-butane, a straight chain isomer, are its two isomers.
Position Isomer/Regio Isomers
Position isomerization happens when two or more compounds vary in the position of a component atom or functional group on the carbon skeleton.
- Regio isomers are another name for positional isomers. The functional group is attached to the hydrocarbon chain at various points. As a result, the molecule has various branching patterns.
- The compound’s number corresponds to the carbon atom with the functional group’s lowest carbon number.
![Position Isomers [Types Of isomers]](https://scienceinfo.com/wp-content/uploads/2023/08/image-176.png)
Functional Isomer
Two or more molecules with the same chemical formula but distinct functional groups are referred to as functional isomers.
- These structural isomers feature distinct functional groups as well as physical and chemical characteristics.
- It refers to compounds that have the same chemical formula but distinct functional groups linked to them, as the name implies.
![Functional Isomers [Types of isomers]](https://scienceinfo.com/wp-content/uploads/2023/08/image-177.png)
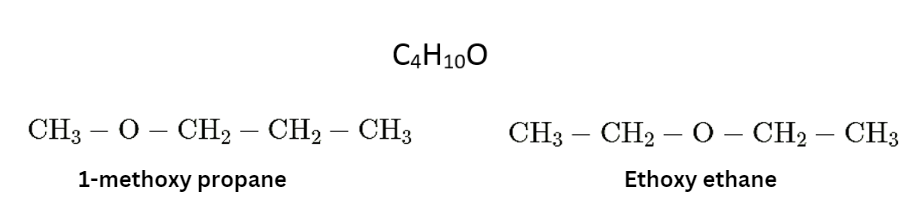
Metamerism
- The existence of distinct alkyl chains on each side of the functional group causes this form of isomerism.
- It is a rare sort of isomerism that occurs only in compounds containing a divalent element (such as sulfur or oxygen) surrounded by alkyl groups.
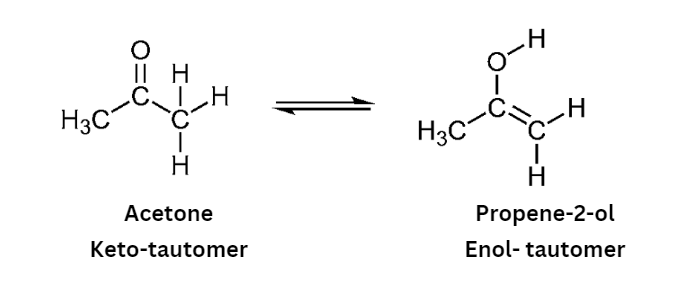
Tautomerism
A dynamic equilibrium between two substances having the same chemical formula is known as tautomerism. A compound’s tautomer is an isomer that only differs in the placement of its protons and electrons. The tautomer of a molecule often coexist in equilibrium and are readily interchangeable. An intramolecular proton transfer causes it to happen. An intramolecular proton transfer causes it to happen. Keto-enol tautomerism is the most prevalent kind of tautomerism.
- In order for an aldehyde or ketone to exhibit tautomerism, α-hydrogen, which is involved in 1,3 – migration, must exist.
- Tautomerism is absent when α-hydrogen is missing.
- For instance, acetone, acetaldehyde, and acetophenone may exhibit tautomerism because they include a hydrogen atom, but benzaldehyde and benzophenone do not since they do not.
Ring Chain Isomerism
Ring chain isomerism is a structural isomerism distinct from the other structural isomers previously addressed. It may be found in both isomeric open-chain and cyclic compounds.
- One of the isomers has an open-chain structure whereas the other has a ring structure in ring-chain isomerism. They typically differ in the quantity of pi bonds present.
- Propene and cyclopropane, for example, are ring chain isomers.

Stereoisomers
Atomic connection is the same for stereoisomers, but their spatial arrangements change. Atoms and functional groups are positioned geometrically differently. Each structural isomer is represented by a different stereoisomer of a molecule.
- This sort of isomerism occurs in compounds with the same chemical formula but various orientations of the atoms in three-dimensional space.
- Stereoisomer molecules are frequently referred to as stereoisomers. Stereoisomers are classified into various classes.
Stereoisomerism is classified into two types:
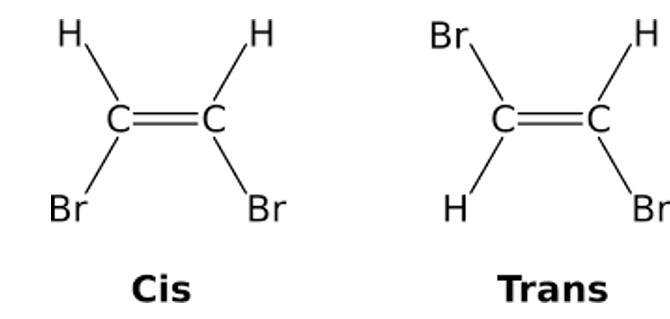
Geometric Isomerism
Geometric isomers feature various configurations of atoms in space but the same order of bonding. A stiff, nonrotatable structure is required for the existence of a geometric isomer. Alkenes and ringed structures show these characteristics.
- There must be two distinct groups connected to each of the two carbons in the double bond.
- Geometric isomerism is sometimes referred to as E-Z isomerism or cis-trans isomerism.
- The physical and chemical characteristics of geometric isomers differ. Because just one group is linked to the carbon atoms involved in the triple bond, alkynes do not exhibit geometric isomers.
Optical Isomerism
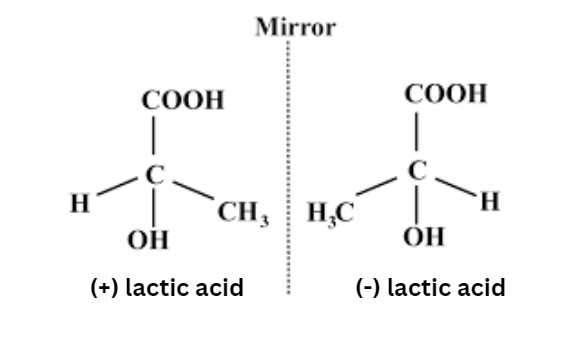
Those stereoisomers that are not geometric are referred to as optical isomers. They differ in how functional groups are arranged around one or more atoms in the main chain. They got their name from their influence on plane-polarized light.
- Compounds with optical isomerism have comparable bonds but distinct spatial arrangements of atoms, resulting in non-superimposable mirror images.
- These optical isomers are also referred to as enantiomers.
- The optical activity of enantiomers vary.
- Dextro enantiomers spin the plane of polarized light to the right, whereas laevo enantiomers rotate it to the left.
Frequently Asked Questions (FAQ)
What is isomerism?
Isomers are two or more compounds that have the same molecular formula but differ in their physical and chemical characteristics. This phenomenon is known as isomerism.
Which type of Isomerism is possible in CH3CH=CHCH3?
The potential structures of the given chemical are H3C-CH=CH-CH3 and H2C=CH-CH2-CH3, and the position of the double bond differs in both configurations. As a result, CH3CH=CHCH3 exhibits Positional Isomerism.
What is Ring-chain isomerism?
Ring chain isomers are compounds that have the same chemical formula but have open-chain or cyclic structures. This phenomenon is known as ring-chain isomerism.
Are constitutional isomers and structural isomers the same?
Yes, constitutional isomers are also known as structural isomers.
Video on Types of Isomers
References
- https://www.britannica.com/science/isomerism
- https://byjus.com/jee/isomers-isomerism-structural-isomerism-jee/
- https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/08%3A_Organic_Chemistry_II_-_Stereochemistry/8.01%3A_Types_of_Isomers
- https://www.studysmarter.co.uk/explanations/chemistry/organic-chemistry/isomerism/
- https://www.geeksforgeeks.org/isomerism/

