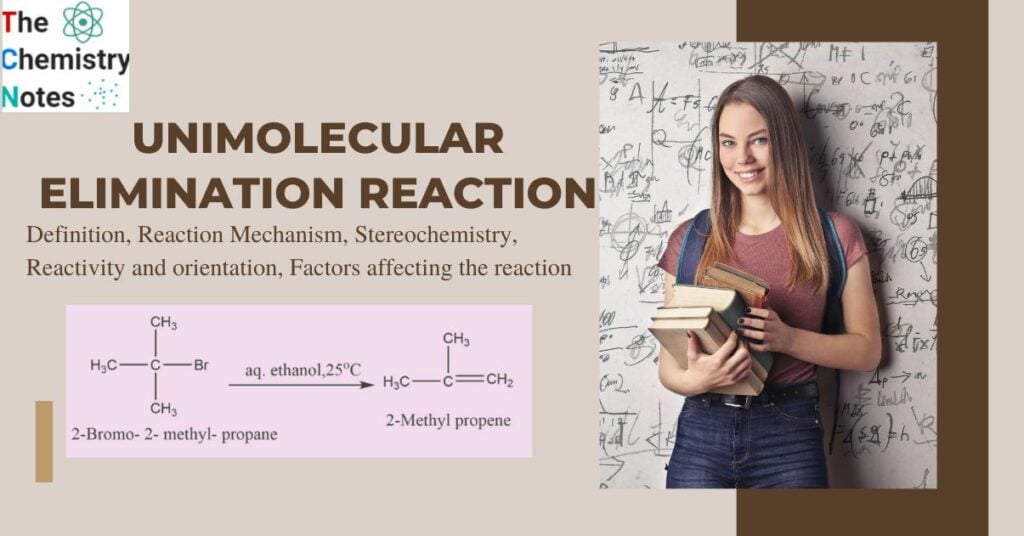
An unimolecular elimination reaction is referred to as an E1 reaction. It is a close analog to the SN1 reaction and the opposite of the electrophilic addition reaction. It is a two-step reaction that typically involves tertiary alkyl halides, though certain secondary alkyl halides may also be involved. When the leaving group is linked to a main carbon, E1 elimination does not take place unless the carbon is in the allylic or benzylic position. Weak bases like water and alcohol generally favor the E1 reaction. For example, 2-Bromo-2- metyl propene in reaction with aqueous ethanol at 25oC produces 2- Methyl propene.

Interesting Science Videos
Mechanism of unimolecular elimination reaction
Step 1: Formation of cation
The reaction starts with the departure of a leaving group and the formation of a carbocation intermediate.

Step 2: Abstraction of hydrogen
When a base removes a proton from a nearby carbon, the vacant p orbital of the carbocation is filled with two electrons, creating a new p bond. The leaving group or another basic species in the solution could serve as the basis for this step.
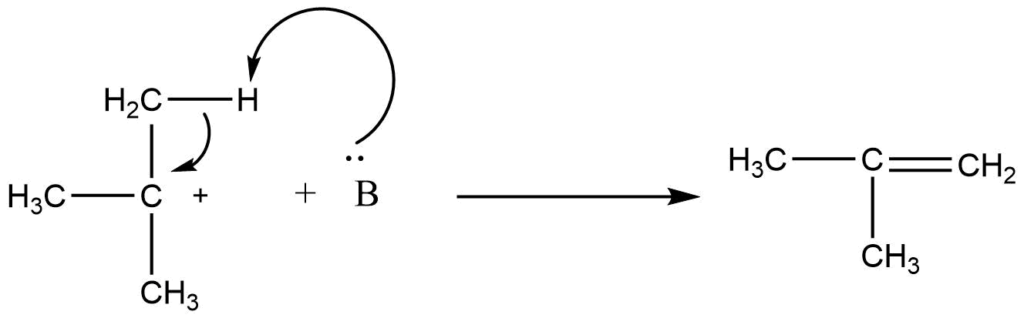
In these reactions, the halogen atom leaves from the α -carbon atom while the base removes a proton from the β-carbon atom, resulting in the formation of a π -bond. So, they are also known as β -elimination reactions.
E1 reactions frequently produce a mixture of various alkene products. The most widely substituted alkene product is the most abundant: This pattern is common in nonenzymatic E1 elimination reactions and is known as Zaitsev’s (Saytzeff) rule, named after Russian chemist Alexander Zaitsev. Both cis and trans alkenes can be produced by E1 reactions. Since trans alkenes tend to be more stable than cis alkenes in general, we can assume that trans alkenes will predominate in the final products.

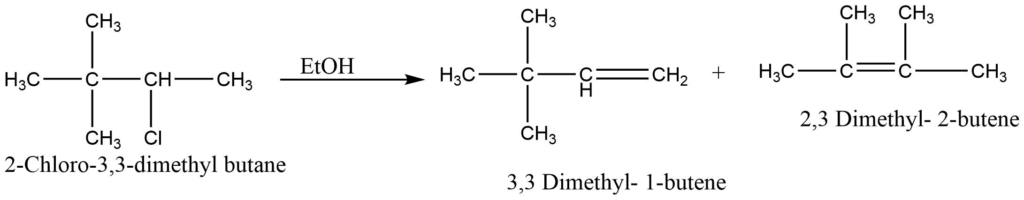
Since a carbocation intermediate is present in the E1 reaction, a carbocation rearrangement may take place if it results in a more stable carbocation. For example, dehydrohalogenation of 2-chloro-3,3-dimethyl butane yields chiefly 2,3 dimethyl- 2-butene as follows,
Kinetics of unimolecular elimination reaction
The reaction follows first-order kinetics.
The reaction is first-order with respect to the substrate and zero-order with respect to the base So the rate of reaction depends on the concentration of substrate only.
rate k = k[(CH3)3 CCl]
Stereochemistry of unimolecular elimination reaction
Since hydrogen and halide are lost in different steps, the E1 reaction does not have a geometric requirement. As a result, the E1 reaction is expected to yield a more stable (Saytzeff rule) product.
Reactivity and orientation of unimolecular elimination reaction
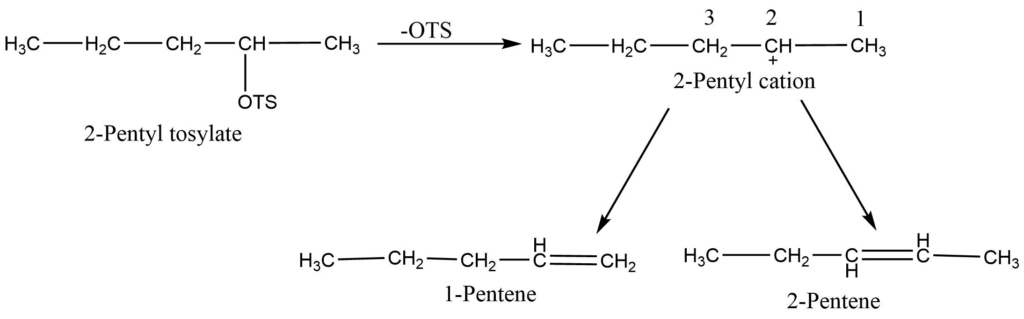
E1 reactions are regioselective. Saytzeff orientation is strongly followed by elimination processes that exhibit the E1 mechanism. The product with the highest degree of branching is favored when more than one alkene can be produced. A disubstituted alkene is therefore favored over a monosubstituted one. For instance, the main product of 2-pentyl tosylate is 2-pentene.

Relative reaction rates of several processes continue to define the orientation and reactivity. The rate of step first determines how quickly the substrate reacts. However, which β -proton leaves the carbocation from second step faster determines which alkene is produced. For example, the 2- pentyl cation can lose either a proton from 2- pentene or a proton from C1 to form 1 pentene. There is competition and more 2- pentene is obtained because 2- pentene is formed faster.
The alkyl halide’s substitution pattern has a significant impact on the rate of reaction. As a result, the rate increases in the following order: tertiary (fastest) > secondary >> primary (slowest). The carbonium ion generated in the first step has the greatest stability, which allows the reaction to be completed, giving rise to the tertiary alkyl halide’s greatest reactivity. The most stable carbonium ion is a tertiary one, followed by secondary and primary. I > Br > Cl > F is the order of the reactivity with various halogens for a particular alkyl group.
Factors affecting the rate of unimolecular elimination reaction
I. Nature of the substrate
Highly substituted alkyl halides typically favor E1 eliminations. The space for the E2 one-step mechanism is constrained by the bulkiness of strongly substituted alkyl halides. Carbocations with high levels of substitution are also more stable than methyl or primary substituted cations. This stability allows the two-step E1 mechanism to operate.
The stability of the carbonium ions follows the order as 30 > 20 > 10. Thus, the substrate which can form the most stable 30 carbonium ion is the most reactive.
II. Basicity and concentration of base
The E1 or E2 mechanism of a reaction is often determined by the strength of the base. Because E1 reactions do not necessitate the addition of a base, the solvent molecule serves the purpose. Therefore, the E1 reaction is unaffected by the base’s concentration or nature. Generally, weaker bases like H2O and ROH favor this type of reaction.
III. The nature of the leaving group
The type of leaving group has a significant impact on the substrate’s reactivity. The best-leaving groupings are ones that are less basic and more polarizable. Thus, the order of leaving group reactivity of the following sets of species is: H2O > ROH > CH3COO–> OH–
I– >Br– > Cl– >F–
IV. Nature of the solvent
Since the E1 reaction involves ionic intermediates, the carbonium ions, the rate of the E1 reaction increases with the increasing polarity of the solvent. E1 reactions are favored by hydroxylic solvents with low polarity and poor nucleophilicity.
V. Effect of temperature
E1 reaction rate increases with an increase in temperature.
Characteristics of unimolecular elimination reaction
- Unimolecular elimination reaction is a two-step reaction process.
- Reaction exhibits first-order kinetics. The rate of reaction depends on the concentration of substrate only.
- Highly substituted alkyl halide reacts faster than the less substituted alkyl halide.
- Unimolecular elimination reaction is generally favored by weak bases like water and alcohol.
- Presence of a better-leaving group increases the reaction rate.
- Unimolecular elimination reaction is favored by the better leaving group which can stabilize the ionic intermediate.
References
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- http://www.lscollege.ac.in/sites/default/files/e-content/Elimination_reaction.pdf
- https://kpu.pressbooks.pub/organicchemistry/chapter/8-2-e1-reactions/.
- https://www.chem.ucalgary.ca/courses/353/Carey5th/Ch05/ch5-5.html.
- https://www.masterorganicchemistry.com/2012/09/19/the-e1-reaction/.
- https://home.iitk.ac.in/~madhavr/CHM102/Lec13.pdf
