Urea is widely used as nitrogenous fertilizer. It is very soluble and hence quick acting but it can easily wash away. Due to its high nitrogen content, urea is a very significant industrial product. It is extensively used as a fertilizer in agricultural fields. Carbamide, an organic substance with the chemical formula CO(NH2)2, is another name for urea. Two amino groups (-NH2) are linked by a carbonyl functional group (-C(=O)-) to form this amide.
It is an odorless, colorless substance that is extremely soluble in water and almost completely harmless (non-toxic). The primary purposes of urea fertilizer are to supply plants with nitrogen and to encourage the growth of green, leafy plants. In addition to being important for photosynthesis in plants, it can make the plant appear lush. Because urea fertilizers can only supply nitrogen and cannot supply phosphorus or potassium, they are generally utilized for bloom growth and may not be wanted for vertical height.
One of the most popular chemical nitrogen fertilizers is urea, which is also the primary raw material used in the production of NPK fertilizer, which is used to make compound fertilizer. Since urea contains high nitrogen content (46%), it’s of high use and hence is synthesized on a large scale.
Interesting Science Videos
Raw materials for the synthesis of urea
- Liquid ammonia (NH3)
- Liquid carbon dioxide (CO2)
The industry uses Haber’s technology to produce ammonia. Limestone (CaCO3) is broken down to create carbon dioxide (CO2). CaCO3 disintegrates into CaO and CO2 when heated. The breakdown of alkali earth metal carbonate produces carbon dioxide and metal oxide.
Manufacture process of urea
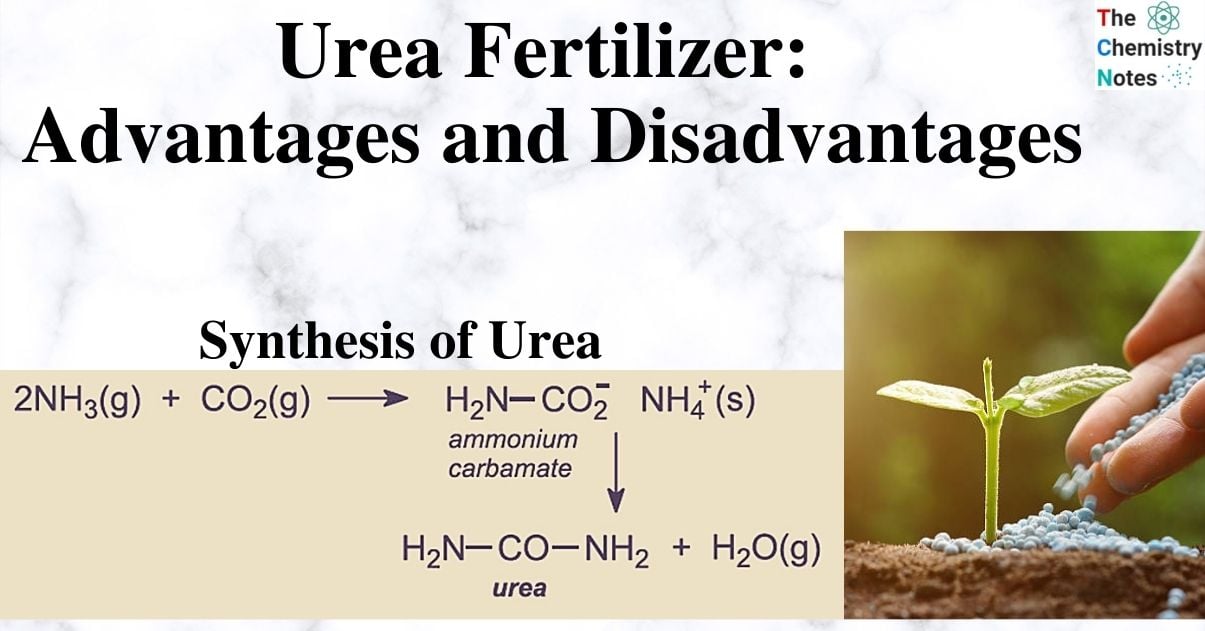
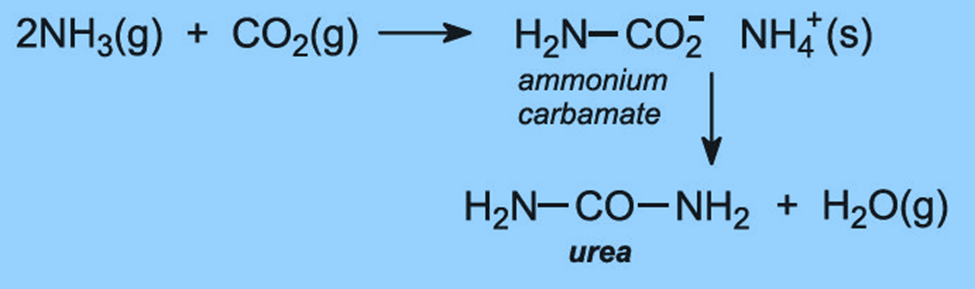
It is manufactured by combining liquid NH3 and CO2 at temperatures ranging from 180 to 200 degrees Celsius and pressures ranging from 100 to 300 atmospheres. Ammonium carbamate, which is unstable and breaks down to produce urea, is first obtained during the two-stage process of urea formation.
In the soil urea slowly hydrolyzes to ammonium carbonate.
NH2CONH2 + 2H2O → (NH4)2CO3
Unreacted ammonia, carbon dioxide, and ammonium carbamate are all present in manufactured urea. Le Chatelier’s Principle states that lowering the pressure will eliminate ammonium carbamate. Ammonia and carbon dioxide are removed from the product mixture during heating. Ammonia and carbon dioxide can be recycled back into the process, which is a benefit of this method. The price of raw materials will go down as a result.
Urea is produced as a solution, but it is then concentrated to produce 99.6% molten urea, which is then ground up and used as fertilizer.
How to use Urea Fertilizer?
- Applying urea should be done right before planting. The seeds shouldn’t come into contact with it. Additionally, it may be used as a top dressing.
- Because urea is so concentrated, it should be mixed with sand or earth before being applied.
When the soil has free water or is anticipated to be moist for three to four days after application, it shouldn’t be applied.
How does urea works in the soil?
Urea is an excellent source of nitrogen and nitrogenous chemicals, and when used properly, it produces good crop yields. Urea dissolves simultaneously when applied as a foliar spray or even as a granular distribution above the soil layer due to its reactivity with both air and water. After being put to the soil, urea goes through critical changes and fragments into ammonium form; the entire process is reliant on soil moisture.
As soon as urea dissolves in the soil, a zoning layer with a high pH and ammonia concentration grows around it, making the soil both toxic and acidic at the same time. Although the majority of soil types neutralize the toxicity to make it harmless. In light of all these consequences, it is strongly advised to spread urea directly into the soil while keeping it away from the seedlings.
Advantages of urea as a fertilizer
- Urea has the highest nitrogen content (46.6%). Higher nitrogen content in urea results in freight and packing.
- High level of nutrients. High levels of nitrogen are readily available; 1 kg of urea is equivalent to 2 kg of ammonium sulfate;
- The cost of production of urea is relatively low.
- It doesn’t change the pH of the soil.
- Urea can be used for all types of soils.
- Urea is not subject to fire on explosion hazards, as in ammonium nitrate and hence there is no risk in the storage of the urea.
- Abundant raw materials. Chemical fertilizers are made from natural mineral resources such as oil, gas, coal, phosphate rock, and other raw materials. These raw resources are abundant and have great potential for use.
- Providing each plant with relevant elements needed, urea sustains plant life.
Disadvantages of urea as a fertilizer
- Urea is very soluble in water and hygroscopic and hence requires better quality package than ammonium sulphate.
- The fact that urea decomposes even at low temperatures, generating NH3 and CO2, makes it less stable than other nitrogenous fertilizers. There is significant loss as a result of NH3 and CO2 production.
- Since the impurities are hazardous to some crops, especially citrus fruits, urea that has impurities in excess of 2% cannot be utilized as a fertilizer.
- It causes increased ammonia concentrations in the soil, which makes it even more acidic and prevents the soil from being naturally fertile.
- During production, hazardous pollutants are released by urea. Pollutant gas emissions from the industrial process enter the atmosphere.
- It is simple to harm fertilizers by using too much urea. Because urea contains a lot of nitrogen, it shouldn’t be used in excess to prevent waste and harm to fertilizers.
- If urea is applied to alkaline soil or soil that has a lot of organic matter, nitrogen will be lost more quickly and completely. Weeds can also easily consume urea.
- Urea must be applied in advance as it takes long period of to start working. When urea is applied as a top dressing, we need to be aware of how it breaks down in the soil. It usually takes urea around 7 days after treatment to transform into ammonia nitrogen, which has the fertilizer effect. Most of the nitrogen will convert to ammonia gas and volatilize in an alkaline environment. Therefore, lime, plant ash, calcium magnesium phosphate, and other common fertilizers cannot be combined with or applied at the same time as urea.
Uses of Urea
- Agriculture: More than 90% of the urea is produced for industrial usage worldwide to use as a nitrogen-release fertilizer. In the soil, urea decomposes to produce ammonium. Losing nitrogenous compounds to runoff and the atmosphere is inefficient and bad for the environment. Because of this, urea may occasionally be pretreated or altered to improve the efficacy of its agricultural application.
- Explosives: A powerful explosive that is utilized in industry and some improvised explosive devices, urea nitrate can be produced from urea.
- Chemical industry: Urea is regarded as one of the most useful materials in chemical industry as it acts as raw materials in the manufacture of numerous plastics, like the Urea-formaldehyde resins, Various adhesives, such as Urea-formaldehyde, and so on.
- Automobile: To minimize the NOx pollutants in exhaust gases from combustion, such as those from power plants and diesel engines, urea is employed in SNCR (Selective Non-Catalytic Reduction) and SCR (Selective Catalytic Reduction) processes.
- Medical use: Topical dermatological products that aim to rehydrate the skin often contain urea. Likewise, Abortions are carried out via urea injections along with various other application in several medical issues.
- Laboratory use: Urea is also highly applicable in the laboratory for several purposes like hydrogen source accompanied by fuel cell power generation, powerful protein denaturant, fixed brain tissue transparent, and so on.
- De-Icer: For de-icing, urea is a secure, non-corrosive fertilizer substitute. The substance is simple to apply on walkways, runways, landing gears, and other crucial components on an aircraft’s undercarriage that must always be kept free from corrosion.
- Used as a part of animal feed that serves as a reasonably affordable source of nitrogen to encourage growth.
- Urea is also applicable as an ingredient in dish soap.
References
- Meessen JH, Petersen H (2010). “Urea”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH
- https://www.fertilizergranulator.com/industry-info/advantages-disadvantages-urea-fertilizer.html
- https://extension.umn.edu/nitrogen/fertilizer-urea
- https://www.britannica.com/science/urea
- https://www.fertilizer-machines.com/solution/market-analysis/functions-of-urea-fertilizer.html
- https://www.indoramafertilizers.com/article/33/urea.html#:~:text=The%20main%20function%20of%20Urea,primarily%20used%20for%20bloom%20growth.
