UV-Vis spectrophotometry is a sophisticated analytical method for measuring light absorption across the ultraviolet (UV) and visible (Vis) ranges of the electromagnetic spectrum that is applied in many different scientific domains. A UV-Vis spectrophotometer measures the amount of light that enters a sample solution and compares that intensity to the light that was incident, providing important insights into the characteristics of materials and how they interact with light.
Interesting Science Videos
What is a UV-Vis Spectrophotometer?
- UV-vis spectrophotometers are analytical tools that apply the principle of UV-visible spectrophotometry, which examines how light is absorbed in the UV-visible spectrum using substance molecules.
- The UV-Vis spectrophotometer operates on the principle of absorption, which is the process by which light is absorbed by the material. A photon, or particle of light, is absorbed by an atom or molecule, and its energy is transferred to that atom or molecule’s electron. As a result, the electron’s energy level rises, and it is this energy gain that UV-Vis spectroscopy measures.
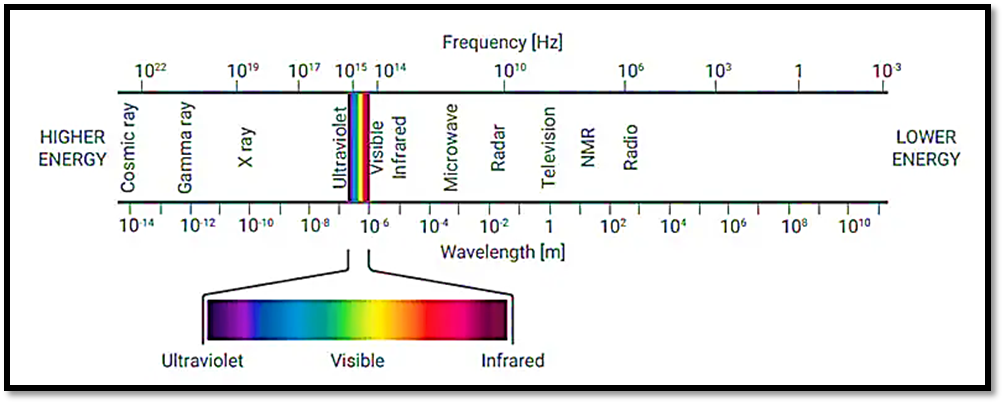
- Using a light source to illuminate a sample with light spanning the UV to the visible wavelength range (usually 190 to 900 nm), ultraviolet-visible (UV-Vis) spectrophotometers work. The light that the sample absorbs, transmits, or reflects at each wavelength is then measured by the instruments. A few spectrophotometers can measure wavelengths up to 3200 nm, or the near-infrared (NIR).
- Diverse measurements can be conducted using a UV-Vis spectrophotometer by utilizing a variety of accessories and sample holders. These accessories cater to different measurement capabilities and sample characteristics, such as distinguishing between solids and liquids, and are designed to accommodate various measurement conditions.
- It is possible to determine the chemical or physical properties of the sample:
- Determine the molecules in a sample that is liquid or solid.
- Find the amount of a specific molecule in a solution.
- Describe the transmittance and absorption through a liquid or solid at various wavelengths.
- Determine a surface’s reflectance characteristics or a material’s color.
- Examine biological processes or chemical reactions.
How does the UV-Vis spectrophotometer work?
The molecule’s ultraviolet-visible absorption spectrum is the result of the electronic energy level transitioning after certain molecular groups have absorbed ultraviolet-visible light.
Within the analyte, the absorption of light energy varies due to the distinct molecules, atoms, and molecular spatial structures present in different substances. Spectrophotometric analysis proves to be a valuable approach for investigating the composition, structure, and interactions of substances through their absorption spectra. These spectra, characterized by band-shaped patterns, provide insights into specific groups within the molecules. Qualitative analysis can be enhanced by combining the standard light spectrum with other methods.
According to Lambert-Beer’s law, the absorption of light is directly proportional to the thickness of the absorbing layer. Beer’s law further states that light absorption is proportional to the concentration of the solution. Considering both the thickness of the absorbing layer and the impact of solution concentration on light absorption, Lambert Beier’s law is expressed as
A=εbc, where A represents absorbance, ε is the molar absorption coefficient, b is the thickness of the liquid sample, and c is the solution concentration.
This formulation enables the quantitative analysis of solutions. To perform the analysis, the sample and a standard specimen are prepared in the same solvent at equivalent concentrations. Their ultraviolet-visible absorption spectra are separately measured under identical conditions. If the substances in both samples are identical, their spectra should match. In the absence of a standard sample, a comparison can be made with a pre-established standard spectrum. The accuracy and precision of the spectrophotometer are crucial, and consistent measurement conditions are essential for reliable results.
In simple, it can be summarized as:
- Light can be absorbed, reflected, or transmitted when it strikes an object.
- The intensity of light absorbed in the UV and Vis regions is measured using the spectrophotometer.
- A reference measurement of the incident light source is used to compare the amount of light passed through the sample.
- The spectrophotometer can determine the concentration of particular analytes in the sample by using the Beer-Lambert Law, which stipulates that the amount of light absorbed is exactly proportional to the concentration of the sample and the path length.
What does a UV-Vis spectrophotometer measure?
The light that is absorbed, transmitted, or reflected by the sample across a specific wavelength range is measured by UV-Vis and UV-Vis-NIR equipment. Because the Beer-Lambert law described the linear connection between concentration and absorbance, absorbance (A or Abs) is commonly measured in UV-Vis spectroscopy. The fraction of light transmitted or absorbed might have greater significance for other uses. For example, it might be more beneficial to compare the absorbance difference or the percentage of transmission when comparing the optical qualities of different materials.
When reporting UV-Vis observations, most do so to wavelengths expressed in nanometers (1 × 10−9 m). The reciprocal length, often known as the wavenumber (cm-1), is used in some older literature. Wavelength is typically favored for UV-Vis spectroscopy because it provides an easy means of visualizing the displayed spectrum across a spectral range. The majority of UV-Vis spectrophotometers allow you to obtain a spectrum in either wavelength or wavenumber format.
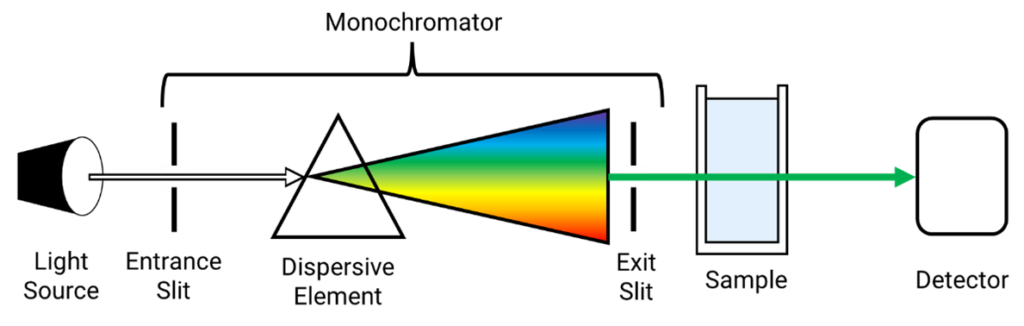
Components of UV-Vis Spectrophotometer
- Light Source: This apparatus offers incident light that satisfies the specifications. Gas-discharge light sources and heat radiation light sources come in two varieties. Gas-discharge light sources are used in the ultraviolet region, usually hydrogen and deuterium lamps, with a continuous wavelength range of 180~360nm; thermal radiation light sources are used in the visible light region, generally tungsten lamps and tungsten halogen lamps, with a wavelength range of 350~1000nm.
- Monochromator: Its purpose is to separate the necessary monochromatic light beam and break down the composite light produced by the light source into monochromatic light. It is the spectrophotometer’s central component.
- Absorption Cell: A cuvette is another name for it. The test solution’s absorbance is measured using it. Ground glass makes up its bottom and two sides, while surfaces on the other two sides are optically clear. The absorption cell’s optical surface needs to be fully developed to minimize light reflection loss. in line with the direction of the beam. The material is classified into two categories: quartz cells and glass cells. While the latter is used to measure the ultraviolet light zone, the former is used to measure the visible light region.
- Detector: It is an apparatus for converting optical to electrical signals. To measure absorbance, light intensity is first converted into a current signal for testing rather than being measured directly as light passes through the absorption cell. We refer to this photoelectric conversion mechanism as a detector.
- Signal display system: An apparatus that shows and enhances the signal produced by the detector.
How to use a UV-Vis spectrophotometer?
- The sample is placed within the sample compartment so that it can be illuminated by the monochromator’s beam.
- Liquid samples would normally be kept in a cuvette with a known, fixed pathlength to measure absorbance.
- A cuvette is a rectangular liquid holder. Glass, quartz, plastic, or any other substance that transmits visible or UV light can be used to make them. Standard cuvettes are composed of quartz and have a 10 mm path length to guarantee good UV wavelength transmission. Although less expensive plastic cuvettes are an option, they are typically not suitable for UV light transmission and should only be used for measurements in the visible wavelength range. There are several different types of cuvettes available for specific uses; these range from cuvettes with very long path lengths for use with extremely diluted samples to cuvettes that can handle very small amounts of liquids.
- For straightforward transmission measurements, solid samples can be fixed in place. Additionally, they can be measured at different incidence angles.
- Additional accessories may be placed into the sample container for more intricate measurements, such as diffuse reflectance or transmission.
- Fiber optics can also be used to extract the light from the sample chamber. When measuring highly large, hot, cold, radioactive, or other risky samples, fiber optics come in handy.
- Solutions outside of the sample compartment can be measured using fiber optics by passing the light from the spectrophotometer through a fiber-optic probe.
- An alternative is to employ a fiber-optic instrument that measures the transmission, fluorescence, or reflectance of light through a solid material.
Wavelength range of a UV-Vis spectrophotometer
Using a light source to illuminate a sample with light spanning the UV to the visible wavelength range (usually 190 to 900 nm), ultraviolet-visible (UV-Vis) spectrophotometers work. The wavelength range of UV-Vis-NIR spectrophotometers is expanded into the near-infrared (NIR) region, which spans from 800 to 3,200 nm.
Limit of detection for a UV-Vis Spectrophotometer
Depending on the instrument and experimental conditions, the limit of detection (LOD) for a UV-Vis spectrophotometer usually varies from low microgram per milliliter (μg/mL) to nanogram per milliliter (ng/mL). Selective wavelengths, pre-concentration methods, signal averaging, and high-quality instruments can all help to increase LOD.
Double Beam Spectrophotometer
The sample beam and the reference beam are the names given to the two distinct light paths seen in a double beam spectrophotometer. A double beam spectrophotometer produces more precise and dependable findings by comparing the sample and reference beams because it instantly corrects absorbance variations over time, which is not the case with single beam spectrophotometers.
Single Beam Spectrophotometer
- A single-beam optical system powers the most basic UV-Vis spectrophotometer.
- Light from the monochromator travels through the sample and onto the detector.
- This instrument’s size and cost are decreased because of its straightforward design, which uses fewer optical components.
- However, a baseline, or blank, sample needs to be measured before a sample may be measured.
- The baseline reading is obtained for liquid measurements to account for any solvent and cuvette absorbance. The baseline and the sample must be monitored independently when using a single-beam system.
- The measurement may be less accurate if there is any difference in light intensity or system optical performance between the sample being read and the baseline due to the individual readings.
- Sample measurements that take a long time or where the blank might change over time should be concerned about this inaccuracy.
- In practical terms, this means that if a single beam system is being used, a baseline/blank measurement needs to be performed often and consistently throughout a measurement session.
Double Beam Spectrophotometer
- A double-beam optical system, which divides the light output from the monochromator into a reference beam and a sample beam, is used by many UV-Vis instruments.
- Usually, a beam splitter, which is a half-silvered mirror, or a revolving wheel with a mirrored section is used to split the light.
- The sample chamber is reached via distinct optical pathways for every beam.
- The reference/blank and sample can be measured simultaneously because there are two beams available with the same wavelengths.
- This implies that any instrument fluctuations can be instantly adjusted for in the sample measurement. A very precise measurement is produced by this real-time adjustment.
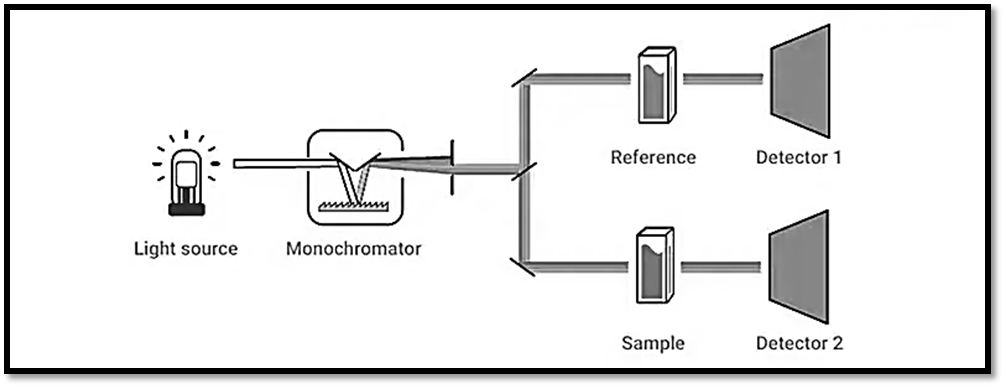
Dual Beam Spectrophotometer
- A sample and reference detector is part of a dual-beam optical arrangement used in a more modern spectrophotometer design.
- While the solvent or blank (in the case of a solid sample) is detected in the sample position and then subtracted from the sample spectrum after collection, the reference detector is used to correct lamp brightness fluctuations for each measurement.
- This design minimizes the possibility of human mistakes caused by mismatched cuvettes or improper sample placement by streamlining the measuring procedure and reducing complexity through advancements in electronics and software.
- While double-beam design is currently usually reserved for research-grade instruments, dual-beam design performs the same as a conventional double-beam instrument.
Applications of UV-Vis Spectrophotometer
UV-Vis spectrophotometry is widely used in many different scientific fields. Among the numerous noteworthy uses are protein and nucleic acid analysis, chemical analysis, and many more. Below are some of the widely used applications:
- Chemical Analysis: Biomolecules, organic chemicals, and inorganic metals are commonly quantified in educational and industrial materials science labs using UV-Vis spectrophotometry. Its simplicity and speed of use make it a preferred method.
- Nucleic acid and protein analysis: UV-Vis spectrophotometry provides precise protein and nucleic acid concentration measurements, which is critical for applications in molecular biology, biochemistry, and genetics. For convenience of usage, many instruments include colors and protein types preconfigured or defined.
- Pharmaceutical research: UV-Vis spectrophotometry is an essential tool in the pharmaceutical business for identifying and quantifying chemicals in pharmaceutical products to ensure their efficacy and purity.
- Purity Testing: DNA samples are evaluated for purity using UV-Vis spectrophotometry, which guarantees that the samples are suitable for downstream processes like PCR and DNA sequencing.
- Microvolume analysis: Modern UV-Vis spectrophotometers are appropriate for small sample volumes because of technological improvements that allow them to analyze microvolume samples as little as 0.5 microliters.
- Quality control analysis: To ensure the quality and uniformity of ingredients and products, the approach is frequently employed in industries including food, cosmetics, and pharmaceuticals.
Advantages of UV-Vis Spectrophotometer
UV-Vis spectrophotometry is a useful analytical method in many scientific domains because of its many benefits.
- Easy to use: UV-Vis spectrophotometry is a useful analytical method in many scientific domains because of its many benefits.
- Non-destructive: UV-Vis can be used on a variety of chemical species and enables non-destructive analysis. Because samples won’t be harmed, this enables repeated studies, which is a crucial advantage for quality assurance and control.
- Rapid analysis: UV-Vis spectrophotometers enable researchers to get data in a matter of seconds by offering quick and effective analysis. It is used for quality control in the pharmaceutical and food sectors as well as to quantify the amount of protein and nucleic acids in biological samples.
Disadvantages of UV-Vis Spectrophotometer
Comparing UV-Vis spectrophotometry to techniques like fluorescence quantification reveals several drawbacks.
- Its reduced sensitivity and selectivity make it less useful in some situations.
- Stray light can have an impact on absorption measurement accuracy, which could have an impact on the accuracy of spectra measurement.
References
- https://www.agilent.com/en/support/molecular-spectroscopy/uv-vis-uv-vis-nir-spectroscopy/uv-vis-spectroscopy-spectrophotometer-basics
- https://www.denovix.com/blog/what-is-a-uv-vis-spectrophotometer/
- https://www.drawellanalytical.com/what-is-a-uv-vis-spectrophotometer/
- https://www.mrclab.com/all-you-need-to-know-about-uv-vis-spectrophotometer
