A bimolecular nucleophilic substitution (SN2) reaction results in a change in the configuration of a chiral center known as a “Walden inversion reaction.” This inversion, also known as optical inversion, may or may not result in a change in the direction of rotation. Walden inversion was named after a German chemist, Paul Walden, who discovered it in 1895.
Interesting Science Videos
What is Walden Inversion Reaction?
The Walden inversion refers to the inversion of configuration at a chiral center during a bimolecular nucleophilic substitution (SN2 reaction). Walden inversion changes the structure of the molecule from one enantiomeric form to another because the molecule can produce two enantiomers around the chiral center.
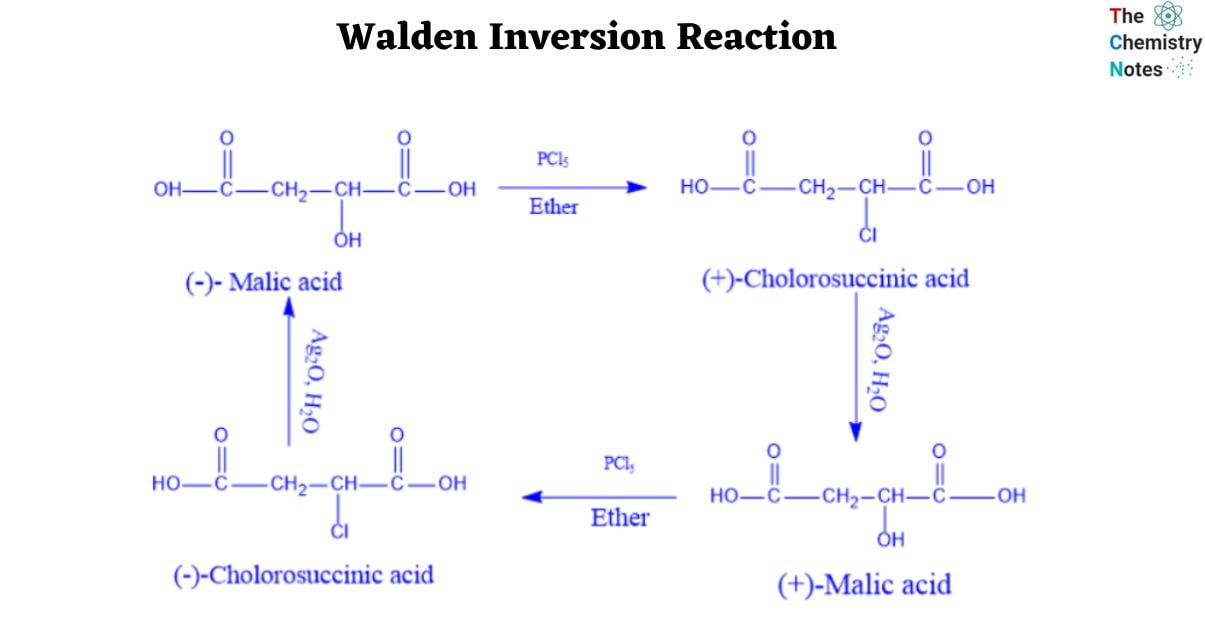
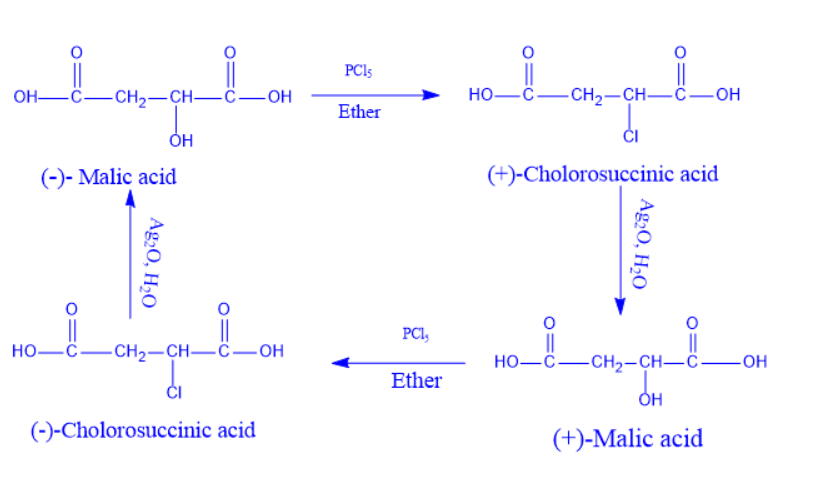
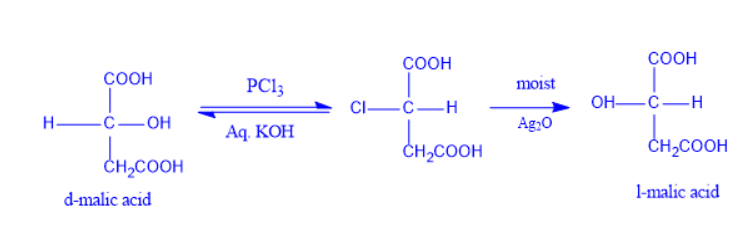
Walden demonstrated in 1986 that (-)-malic acid could be transformed to (+)-malic acid by a series of chemical processes using achiral reagents. This proved that optical rotation is intimately tied to chirality and that it varies with chemical change.
- When (-)-malic acid reacts with PCl5, it produces (+)-chlorosuccinic acid.
- A further reaction with moist silver oxide yields (+)-malic acid.
- The reaction sequence that begins with (+) malic acid ends with (-) malic acid.
Walden Inversion Reaction Example
Walden Inversion Reaction Mechanism
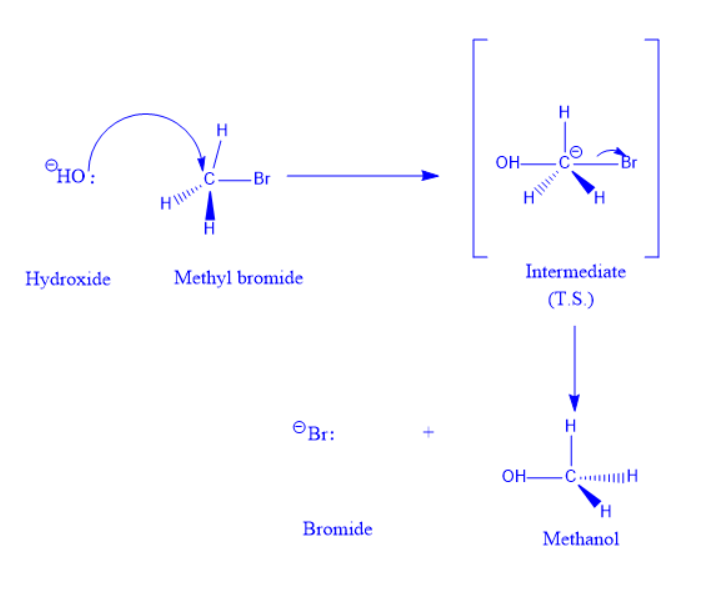
In a chemical process, Walden’s inversion is the reversal of a chiral center in a molecule. Because a molecule can generate two enantiomers around a chiral center, the Walden inversion changes the molecule’s configuration from one enantiomeric form to another.
Walden inversion has a mechanism comparable to an SN2 reaction. An intermediate is formed as a result of the nucleophilic assault on the carbon atom. This is followed by the displacement of the leaving group, which yields the product.
Video on Walden Inversion Reaction
References
- https://byjus.com/chemistry/walden-inversion/#
- https://www.vedantu.com/chemistry/walden-inversion
- https://testbook.com/chemistry/walden-inversion
- https://infinitylearn.com/surge/chemistry/walden-inversion/
- https://www.chemistrylearner.com/walden-inversion.html

