Valence electrons are electrons that exist at the highest energy level of an atom, which is often the outermost level. These electrons are the ones that take part in the bonding process. There is a specific number of electrons in each neutral atom. Neutral atoms always have the same number of electrons and protons (also known as the atomic number).
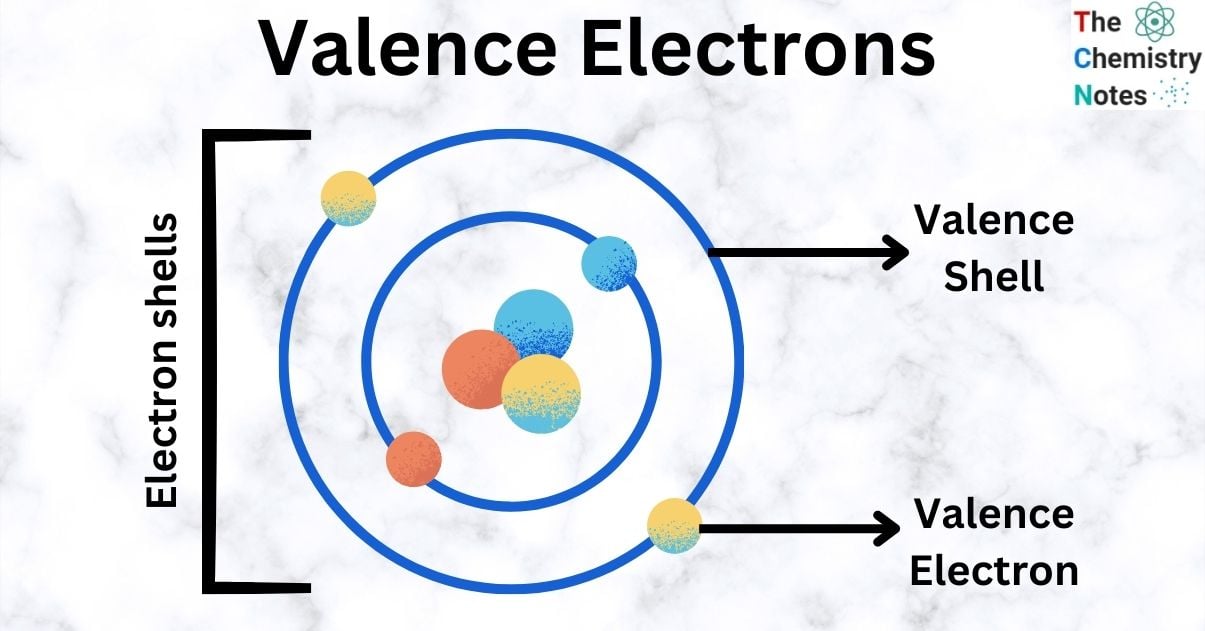
Valence electrons are responsible for the most significant contributions of any of the electrons, even though every electron contributes in some way. Interactions between the valence electrons of two or more atoms can lead to the formation of chemical bonds between the atoms. The ability to form chemical bonds depends on the sharing or donation of valence electrons between atoms.
Interesting Science Videos
What are valence electrons?
Valence electrons are electrons in an atom’s outermost atomic shell that circle the nucleus. These electrons are the ones that take part in bonding and reactions since they are the ones that are located the farthest away from the nucleus and are thus held by the atom the most loosely.
This also implies that the quantity of valence electrons that an element possesses is directly proportional to its reactivity, electronegativity, and the number of bonds that it is capable of forming.
When compared to the electrons that are found in the inner orbits, the valence electrons have a greater amount of energy, which is why they are involved in the chemical processes. The amount of valence electrons that are present is another factor that may be used to define the chemical properties of a particular element. It also indicates how easily atoms may make bonds, the quantity of unpaired electrons, and how many atoms can participate.
What is Valency?
The valency of an element is defined as the total number of valence electrons present in that element. When two or more elements are mixed in a specific ratio, a chemical compound is produced. A stable compound is made when one atom of one element joins with a certain number of atoms of another element. The components vary in their ability to combine.
Significance of Valence Electrons
- Atomic reactivity is directly related to the quantity of valence electron.
- Electronegativity is affected by an atom’s valence electrons.
- An atom’s electron affinity is set by its valence electron.
- Ionization energy is affected by the number of valence electrons in an atom.
- A stable atom is a result of its valence electrons.
- The valence electrons of an atom are what govern its chemical and physical properties.
- Each valence shell of an atom has to be filled with 8 electrons for maximum stability. Hydrogen and helium defy this trend because they are stable with just two electrons in their outermost shell.
- Electrons in a closed shell (inner shell) remain close to the nucleus and cannot escape. The outermost (valence) electrons can take part in bonding by either sharing or migrating to the other atom.
Characteristics of Valence Electrons
- Valence electrons exist only in the outermost electron shells. A transition metal’s inner shell, however, allows for its existence.
- Chemical inactivity is absent from atoms with complete sets of electrons (either octets or duplets).
- They absorb or release energy in the form of tiny energy packets (photons).
- On the basis of electrical conductivity, valence electrons classify elements as metal, non-metal, or metalloid.
How to find Valence electrons of the element?
The number of valence electrons in an element may be determined in two methods.
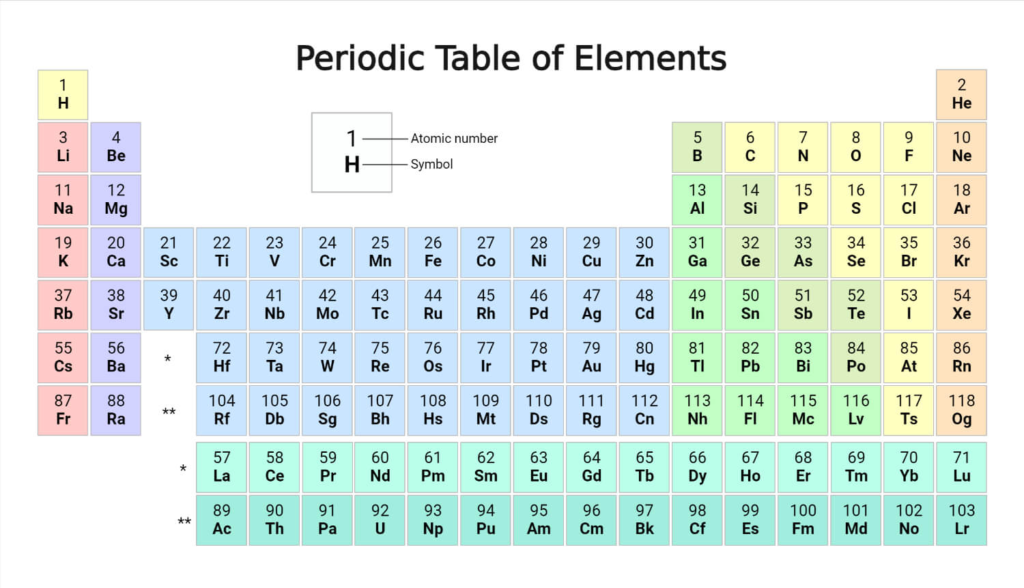
- Using the Periodic Table
In order to count the valence electron of an element, this is the most used technique. Here, we just look up the element in the periodic table to get its specific location. The number of valence electron remains constant even when the number of shells expands as we move from the top to the bottom of a group. On the other hand, going from left to right throughout a period results in an increase of one valence electron.
Note: The number of shells is indicated by the period, whereas the number of valence electron in the outermost shell is indicated by the group. Nevertheless, this only applies to the elements of the two main groups (groups 1 and 2) and groups (13-18). The rule is valid for elements in groups 3–12 that are in transition or an inner transition.
By the Use of Element Electronic Configuration
The orbits of electrons within the shells are known as atomic orbitals. Electronic configuration refers to the specific configuration of these orbitals occupied by electrons. You can quickly get an idea of how many electrons are in the outermost shell by looking at the electron configuration. Hence, we can rapidly calculate the total number of valence electrons in an element by writing down its electrical configuration.
Valence Electrons of Elements in Periodic Table
The electrical configuration and number of valence electron of elements in the second period are tabulated below.
| Name of the element | Electron Configuration | No. of valence electron |
| Lithium | 1s22s1 | 1 |
| Beryllium | 1s22s2 | 2 |
| Boron | 1s22s22p1 | 3 |
| Carbon | 1s22s22p | 4 |
| Nitrogen | 1s22s22p3 | 5 |
| Oxygen | 1s22s22p4 | 6 |
| Fluorine | 1s22s22p5 | 7 |
| Neon | 1s22s22p6 | 8 |
The following table shows the number of valence electron in each group of the periodic table:
| Periodic Table Group | Valence Electrons |
| Group 1 (I) – Alkali metals | 1 |
| Group 2 (II) – Alkaline earth metals | 2 |
| Group 13 (III) – Boron group | 3 |
| Group 14 (IV) – Carbon group | 4 |
| Group 15 (V) – Nitrogen group | 5 |
| Group 16 (VI) – Oxygen group | 6 |
| Group 17 (VII) – Halogens | 7 |
| Group 18 (VIII or 0) – Noble gases | 8 |
Tol learn more about valency and valence electrons, watch this video
References
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General chemistry: principles and modern applications
- https://theimportantsite.com/why-valence-electron-are-important/
- https://www.sciencefacts.net/valence-electron.html
- Miessler G.L. and Tarr, D.A., Inorganic Chemistry (2nd edn. Prentice-Hall 1999)
- Zhou, Mingfei; Frenking, Gernot (2021). “Transition-Metal Chemistry of the Heavier Alkaline Earth Atoms Ca, Sr, and Ba”. Accounts of Chemical Research