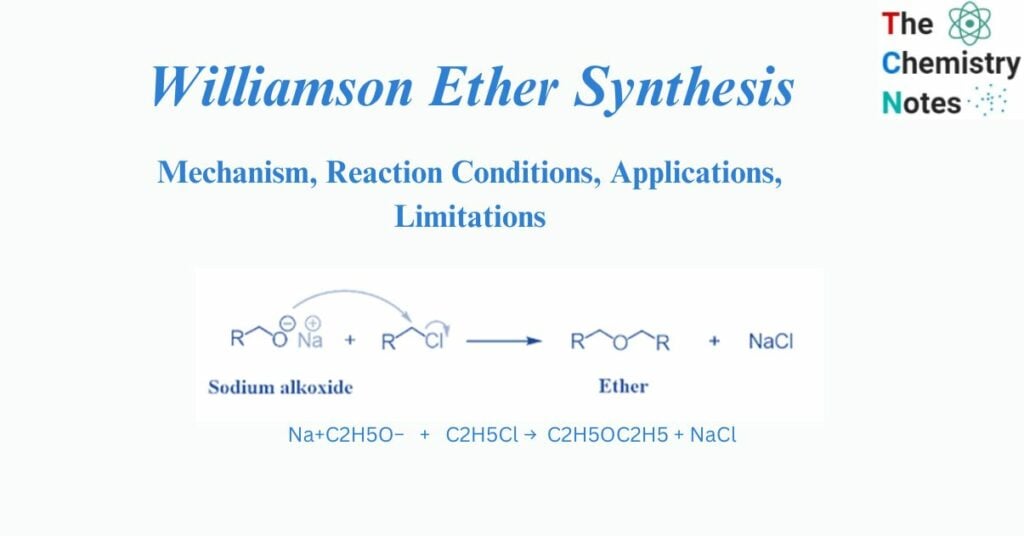
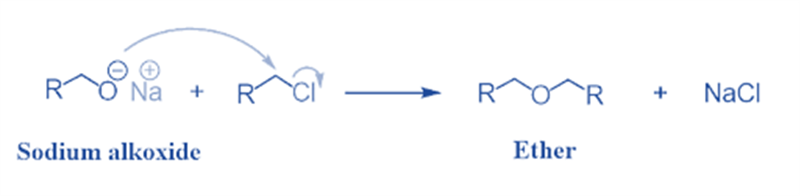
The Williamson ether synthesis is an organic process that involves the reaction between a deprotonated alcohol (alkoxide) and an organohalide to produce ether. Alexander Williamson invented this reaction in 1850.
Typically, it entails an SN2 reaction between an alkoxide ion and a primary alkyl halide. Due to its role in establishing the ethers’ structure, this reaction has played a significant role in the history of organic chemistry.
It is a significant approach in laboratories for producing symmetrical and asymmetrical ethers. An alkyl halide is reacted with sodium alkoxide in this technique, resulting in the production of ether.
Na+C2H5O− + C2H5Cl → C2H5OC2H5 + NaCl
Interesting Science Videos
Reaction Mechanism of Williamson ether synthesis
- An SN2 bimolecular nucleophilic substitution mechanism governs the Williamson ether synthesis reaction.
- An alkoxide ion (RO ) acts as the nucleophile in the Williamson ether reaction, attacking the electrophilic carbon with the leaving group, which is typically an alkyl tosylate or an alkyl halide.
- In the SN2 reaction mechanism, a nucleophile attacks an electrophile from the back.
- One concentrated mechanism allows the entire process to happen at once.
- Strong electronegative properties of halides are required for an SN2 reaction to occur.
- In the Williamson ether reaction, an alkoxide ion (RO) serves as the nucleophile, attacking the electrophilic carbon with an alkyl halide or an alkyl tosylate.
- The tertiary and secondary alkyl groups are more reactive towards an elimination reaction. The reaction’s spatial restriction favors the synthesis of alkenes over the formation of significant ethers like di-tert-butyl ether. Hence primary alkyl halide is usually preferred.
Reaction Conditions for Williamson ether synthesis
- Due to their high reactivity, alkoxide ions are frequently created in situ or prepared just before the reaction. While phase transfer catalysis is frequently used in industrial syntheses, carbonate bases or potassium hydroxide are most frequently used to achieve in situ production in laboratory chemistry.
- A broad variety of solvents can be utilized, however protic and apolar solvents tend to significantly slow down the reaction rate by reducing the availability of the free nucleophile. Acetonitrile and N, N-dimethylformamide are particularly frequently utilized as a result.
- In extreme situations, silver oxide, a silver salt, helps the departing halide group to facilitate its departure.
- Usually, laboratory syntheses do not require catalysis. The rate of reaction can be significantly increased by adding a catalytic amount of a soluble iodide salt, which performs halide exchange with the chloride to produce a considerably more reactive iodide if an unreactive alkylating agent is used (for example, an alkyl chloride).
- The process lasts for approximately 1 to 8 hours and occurs at a temperature of 50 to 100 °C.
- In the lab procedure, a yield of 50 to 95 % is possible because side reactions make it unlikely to completely use up the raw material.
Applications of Williamson reaction
- The Williamson ether synthesis reaction is the simplest and most commonly used technique for making ethers. It has a wide range of applications and is frequently employed in both laboratory and commercial synthesis. It is simple to prepare symmetrical and asymmetrical ethers. Epoxides are produced via the intramolecular interaction of halohydrins.
- The indirect production of ether from two alcohols using the Williamson ether synthesis is another common use. The reaction between two alcohol molecules occurs after one of the alcohols is first changed into a leaving group (often tosylate).
- Alkoxides (or aryloxides) can be primary, secondary, or tertiary. The primary alkylating substances are more preferably used. Secondary alkylating compounds also react, although tertiary ones are frequently too susceptible to side reactions to be useful.
Limitations of Williamson ether synthesis
- The Williamson ether synthesis reaction frequently competes with base-catalyzed alkylating agent elimination, and the type of the leaving group, as well as the reaction circumstances (especially the temperature and solvent), can have a considerable influence on which is preferred. Some alkylating agent structures, in particular, are highly susceptible to elimination.
- In the presence of the alkoxide, which serves as both a base and a nucleophile, tertiary alkyl halides or sterically hindered primary or secondary alkyl halides frequently undergo E2 elimination.
- In addition to the anticipated O-alkylation, alkali phenoxides may also undergo C-alkylation.
- This method of preparing ethers is far too limited to be useful for synthetic organic chemists.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- https://byjus.com/chemistry/williamson-ether-synthesis/
- https://www.chemistrylearner.com/williamson-ether-synthesis.html
