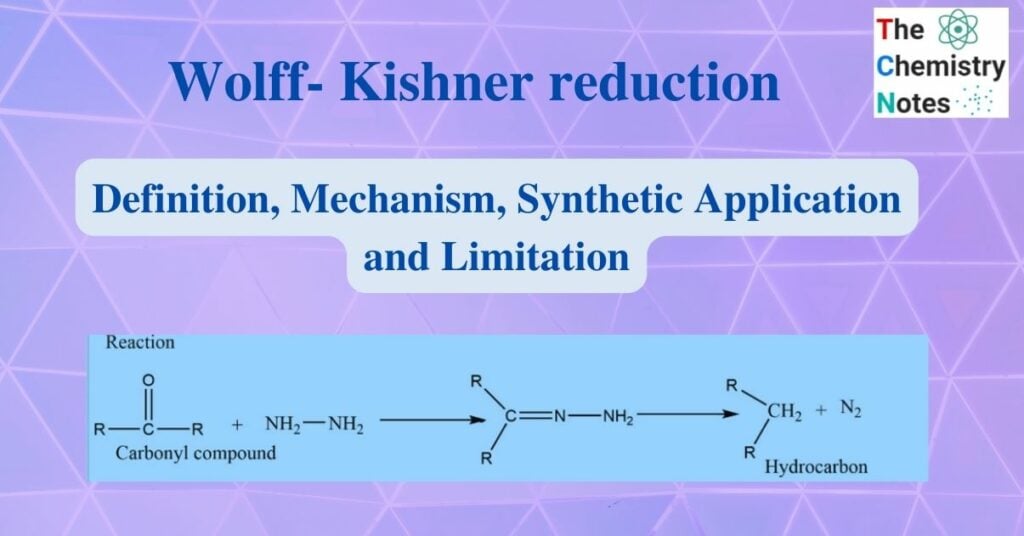
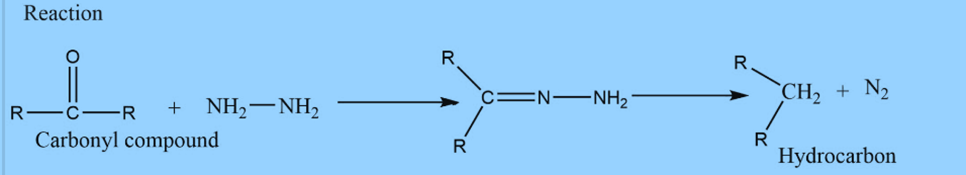
Wolff-Kishner reduction was discovered by Nicolai Kishner in 1911 and Ludwig Wolf in 1912. It involves heating carbonyl compounds with hydrazine and base to convert them to hydrocarbons. In this reaction, aldehyde and ketone react with hydrazine to produce hydrocarbon in presence of base.
Interesting Science Videos
Mechanism of Wolff Kishner reduction
Step 1: The electrophilic addition of hydrazine to C=O to produce hydrazone.
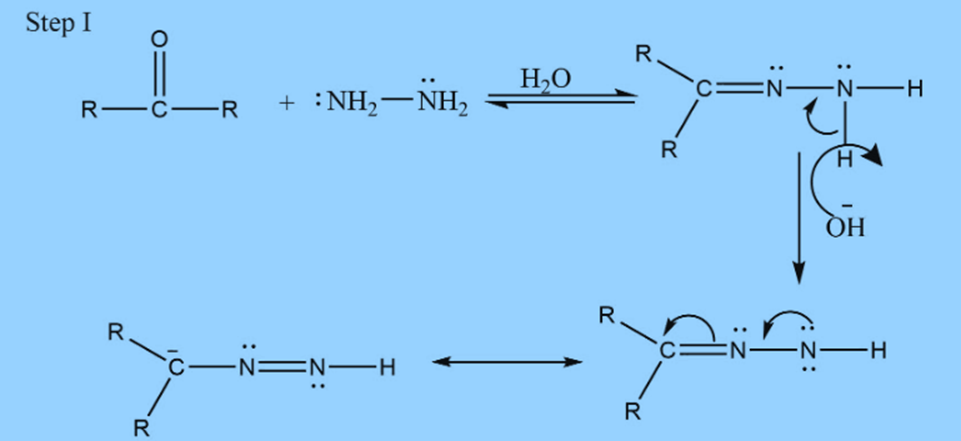
Step II: Addition of base
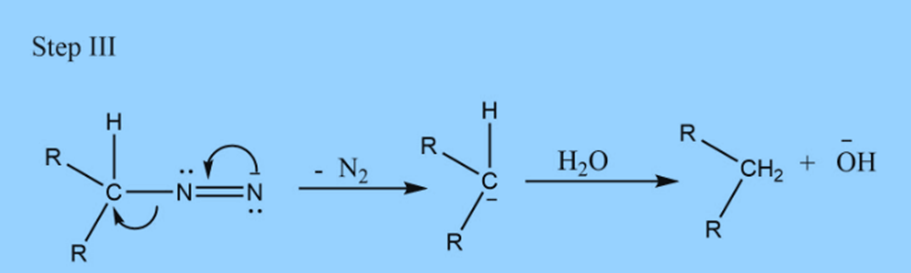
Applications of Wolff Kishner reduction
Wolff Kishner reduction is used for,
- Production of hydrocarbon from carbonyl compounds
- Synthesis of long-chain alkyl chain to the benzene ring
- Polycyclic aromatics and aromatics with side hydrocarbon chains
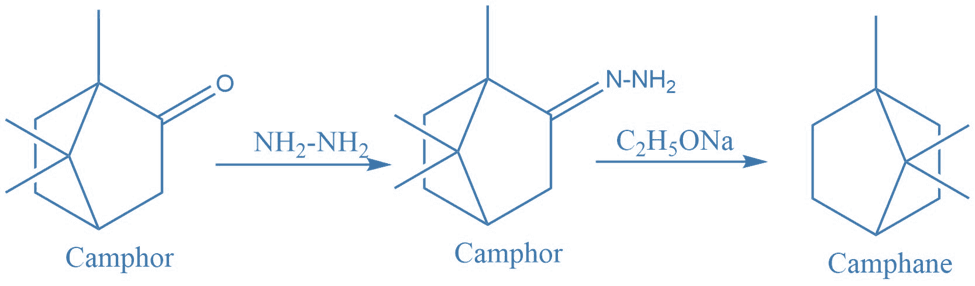
Some examples of sythetic applications
I. Synthesis of camohor from camphane
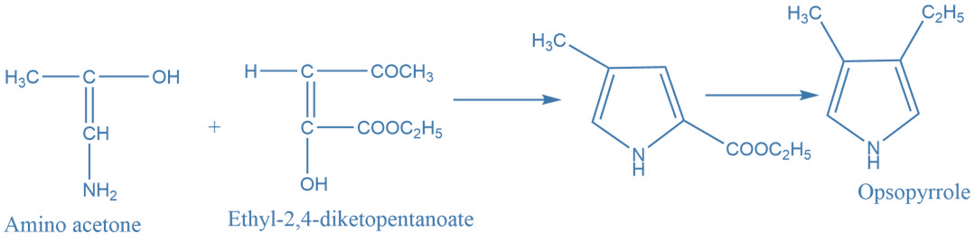
II. Synthesis of pyrrole
Limitations
- Wolff-Kishner reduction reaction needs high temperature.
- Sometimes double bond migration occurs instead of the expected mechanism of the reaction to give undesirable products like alpha, and beta-unsaturated carbonyl compounds.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
