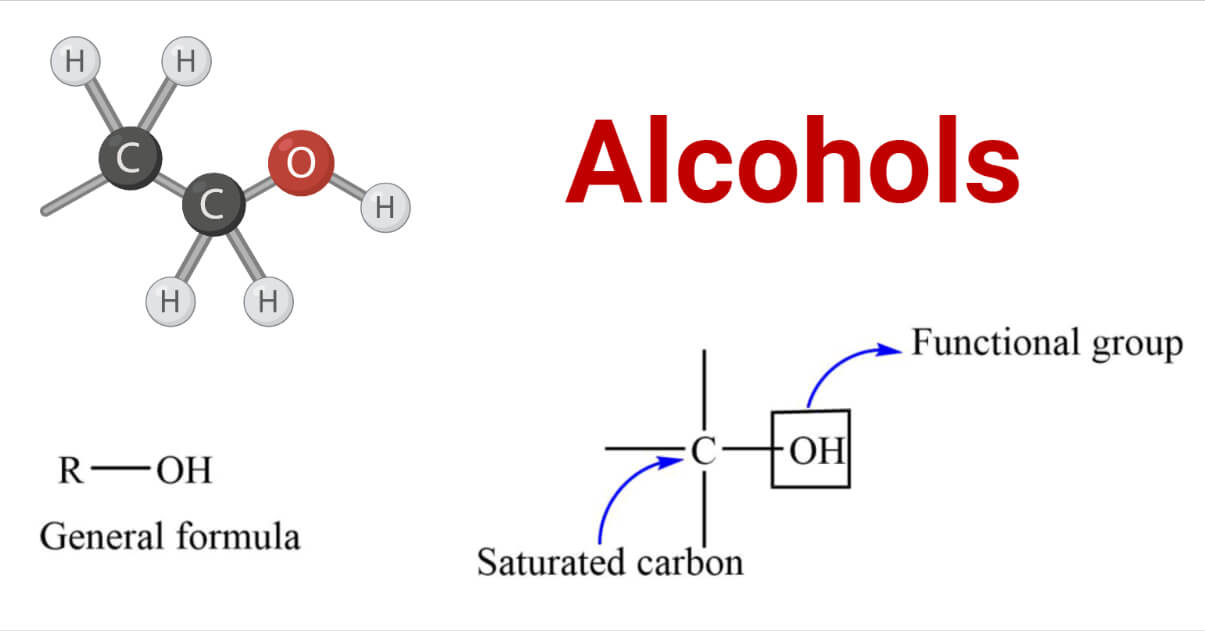
Alcohols are the organic compounds containing the hydroxyl (-OH) group attached to the saturated carbon. The -OH group acts as a functional group of alcohols that determines the characteristic properties of alcohols.
They have the general formula R-OH. where R= alkyl or a substituted alkyl group. The rate of the reaction is affected by the different structures of the alkyl group.
Interesting Science Videos
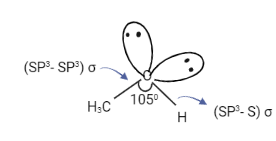
Alcohols Structure
For eg: in methanol, both carbon and oxygen are SP3 hybridized. The C-O bond is formed by the overlapping of SP3 hybrid orbitals of carbon and oxygen while the O-H bond is formed by the overlapping of the SP3 hybrid orbital of oxygen and S orbital of hydrogen. Two completely filled SP3 orbitals of oxygen cannot take part in bond formation.
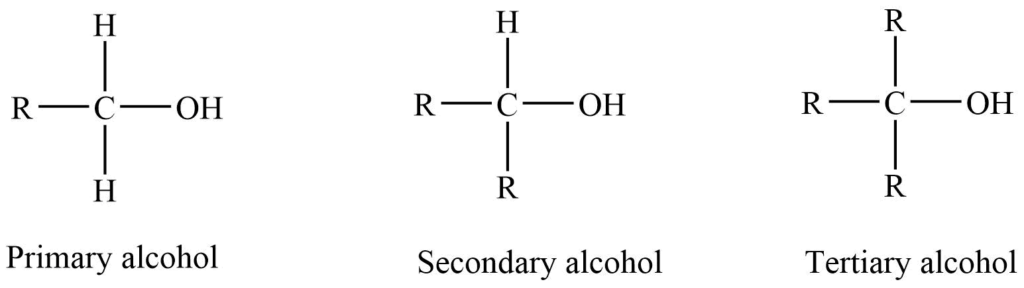
Classification of Alcohols
Monohydric alcohols are classified as primary (10), secondary (20) and tertiary (30) depending upon whether the -OH is present on primary, secondary, and tertiary carbon.
Alcohols containing more than one -OH group are classified as:
Dihydric alcohols: Alcohols with two hydroxyl groups e.g., glycol
Trihydric alcohols: Alcohols with three hydroxyl groups e.g., glycerol
Polyhydric alcohols: Alcohols with more hydroxyl groups e.g., sorbitol
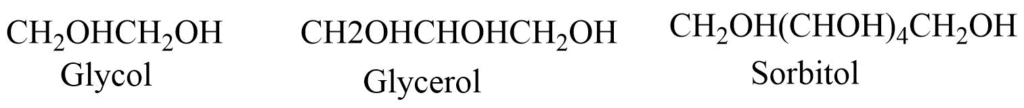
Nomenclature of Alcohols
1. Common name system
In this system, the alkyl group attached to the -OH group is named first, and alcohol is added as a separate word later.

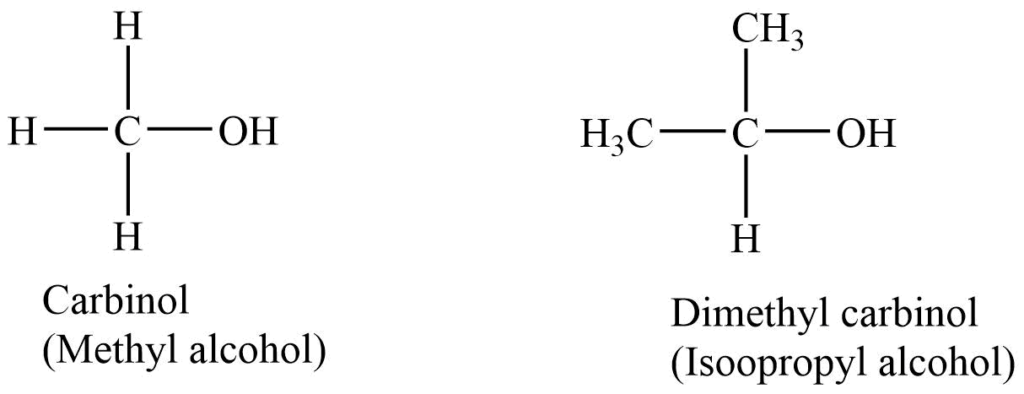
2. Carbinol system
In this system alcohols are treated as methyl alcohol (i.e., carbinol) derivatives. The alkyl groups with the -OH group are named alphabetically, and the suffix -carbinol is added.
3. IUPAC System
- Select the longest chain carbon-containing the -OH group
- Number the chain so that the carbon-containing -OH atom gets the lowest number.
- Name the -OH group and indicate the position of -OH by numbers.
- Indicate the position of other substituents by the numbers and assign an appropriate name to them.
- Prefix their name to the name of the parent chain.
- Replace -e with -ol in the name of the parent chain alkane.
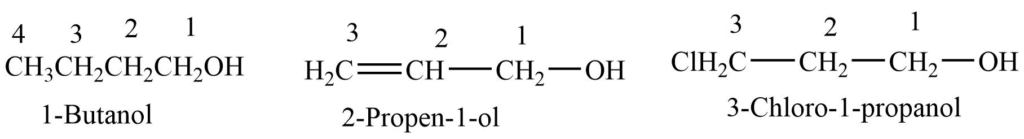
General methods of preparation of Alcohols
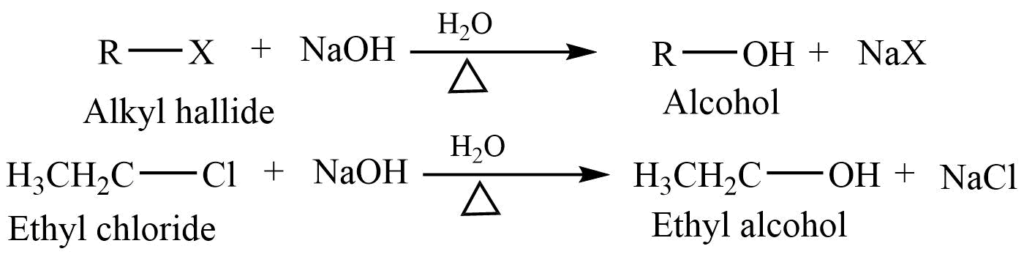
1. Hydrolysis of alkyl halides
Alkyl halides on reaction with aqueous sodium hydroxide gives alcohol.
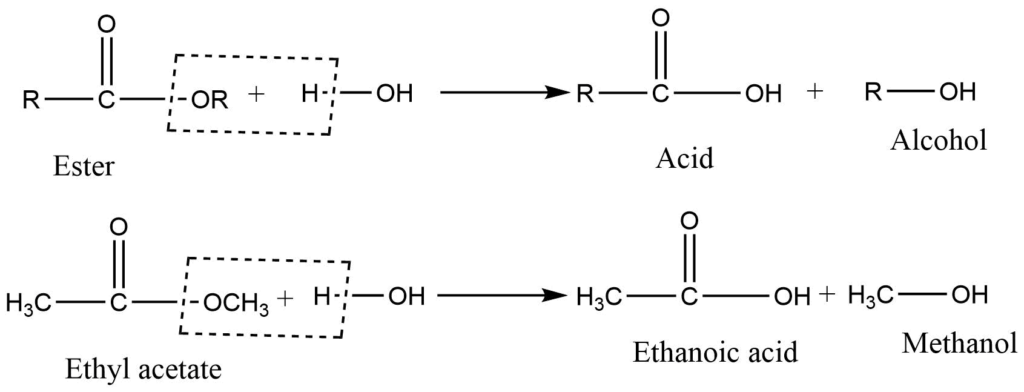
2. Hydrolysis of ester
Acid or base-catalyzed hydrolysis of an ester gives alcohol.
3. Grignard synthesis
Grignard reagent on reaction with aldehydes or ketone gives addition product which on hydrolysis gives alcohol.
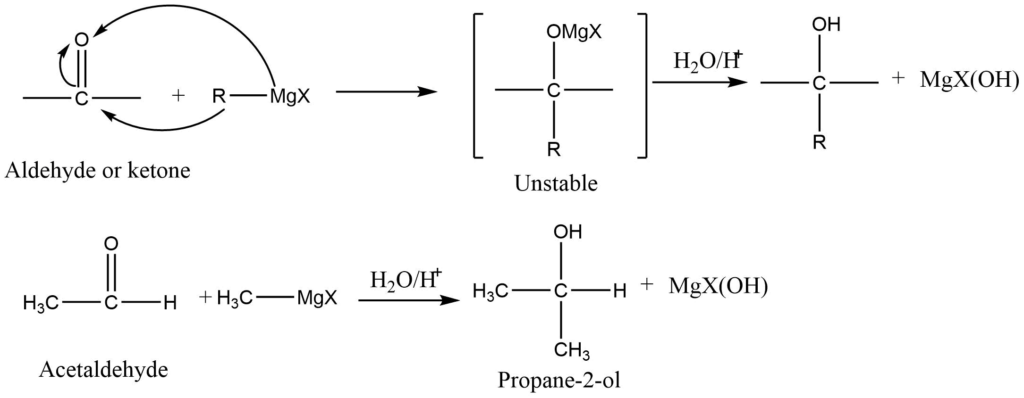
4. Reduction of aldehydes and ketones
Aldehyde and ketones on reduction with H2/Ni lithium aluminium hydride give alcohol. Aldehyde gives primary alcohol while ketone gives secondary alcohol.
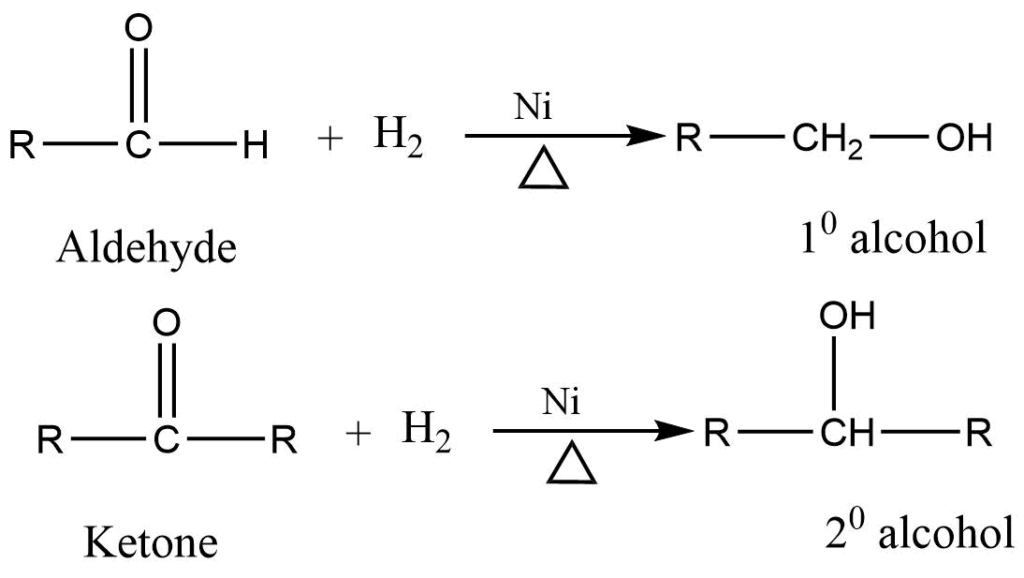
5. Oxymercuration -demercuration reaction
In the presence of sodium borohydride, an alkene reacts with mercuric acetate in an aqueous solution to form alcohol.
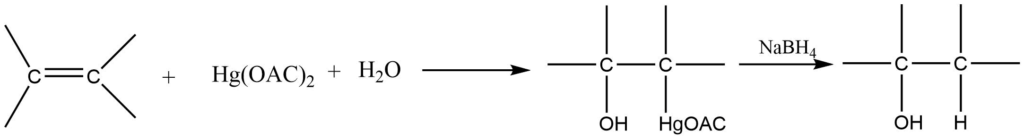
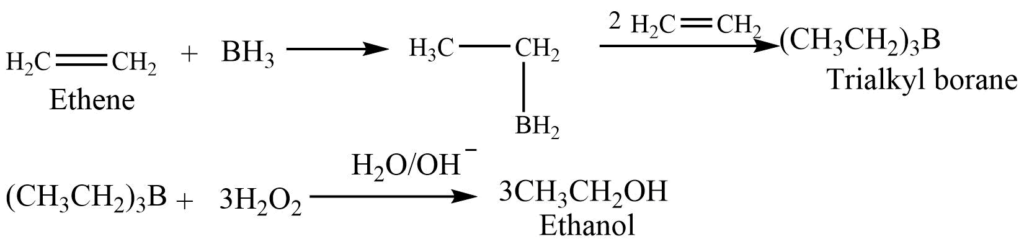
6. Hydroboronation oxidation reaction
Diborane reacts with an alkene to give trialkyl borane. Trialkyl borane on oxidation with hydrogen peroxide gives alcohol.
7. Hydration of alkene
Alkene on reaction with sulphuric acid gives alkyl hydrogen sulphate, which on hydrolysis gives alcohol.
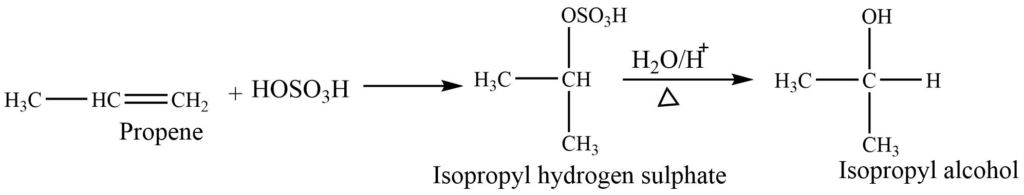
Industrial preparation of alcohols
Alcohols are commonly used as the starting material for several aliphatic organic synthesis reactions. They are also used as a solvent. Industrially alcohol can be prepared from the following methods:
- By hydration of an alkene
- Using the oxo process
- Thorough fermentation of carbohydrate
Physical Properties of Alcohols
- Lower members of alcohols are colorless liquids having a characteristic smell. they are toxic.
- The boiling point of alcohol increases with an increase in the number of carbon atoms.
| Name | Structure | No. of carbons | Boiling point 0C |
| Methanol | CH3OH | 1 | 64.5 |
| Ethanol | CH3CH2OH | 2 | 78.3 |
| Propanol | CH3CH2CH2OH | 3 | 97.0 |
- Due to the presence of the hydroxyl group they are capable of hydrogen bonding.
- The boiling point of alcohols is higher than that of corresponding alkanes.
- The boiling point of isomeric alcohols decreases as branching increases.

- Lower alcohols are soluble in water.
- Due to the presence of -OH stretching alcohols shows a strong absorption band at 3200-3600 cm-1 in the IR spectrum.
Chemical Properties of Alcohols
Alcohols are reactive compounds and can be attacked by polar and ionic reagents.
Some reactions are:
1. Reaction with hydrogen halide
Alcohol in reaction with hydrogen halide produces corresponding alkyl halide.
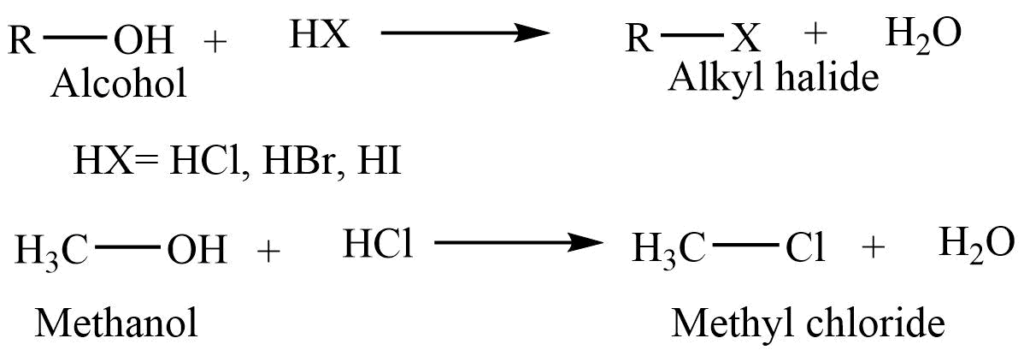
2. Reaction with phosphorous halides
The reaction of alcohol with phosphorous petahalide (PX5) or phosphorous trihalide (PX3) gives corresponding alkyl halide.
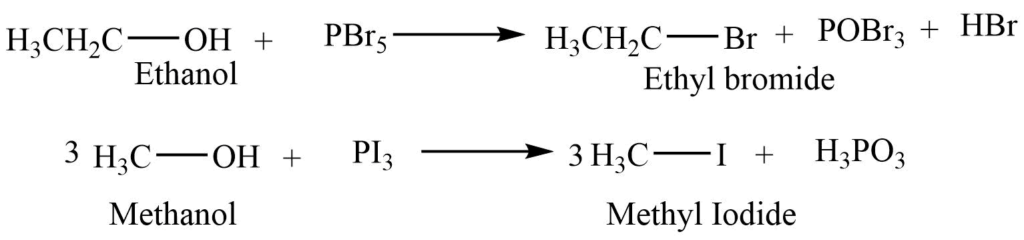
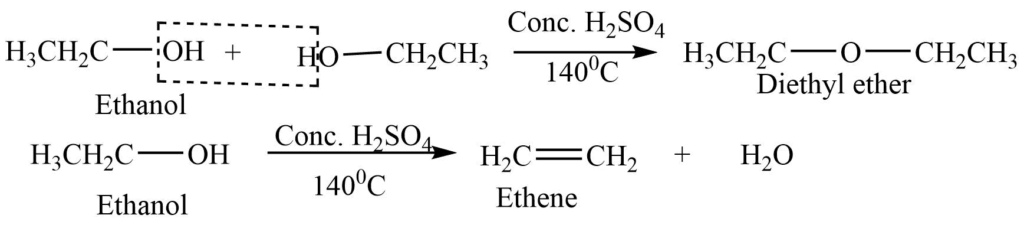
3. Dehydration of an alcohol
Excess ethyl alcohol reacts with concentrated sulphuric acid at 1400C to form diethyl ether, while ethyl alcohol in reaction with concentrated sulphuric acid at 1700C gives alkene.
4. Formation of ester (Esterification reaction)
Alcohols react with a carboxylic acid in presence of concentrated sulphuric acid to give ester. This reaction is a reversible one.
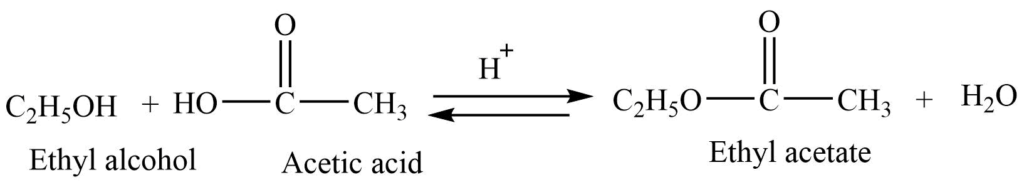
5. Oxidation reaction
Alcohols undergo an oxidation reaction in presence of different oxidizing agents like KMnO4+ H2SO4, Na2Cr2O7 + H2SO4, or K2Cr2O7. The type of alcohol and reaction conditions determine the nature of the product.
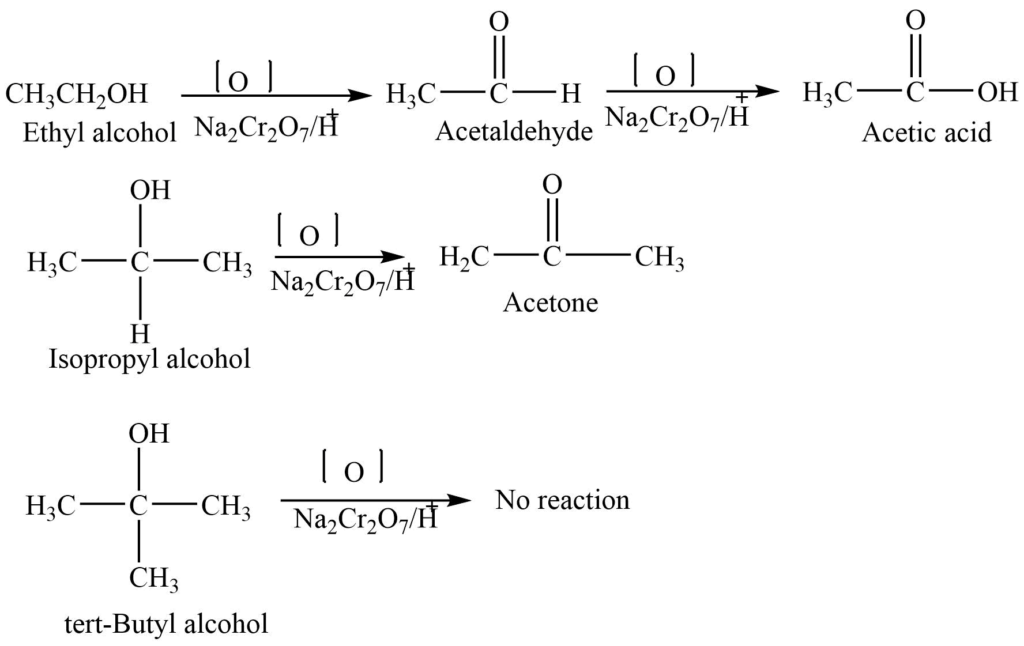
6. Reduction rection
Alcohol on reaction with concentrated hydriodic acid and red phosphorus reduces to give an alkene.
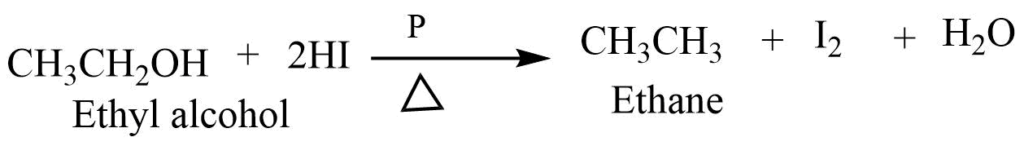
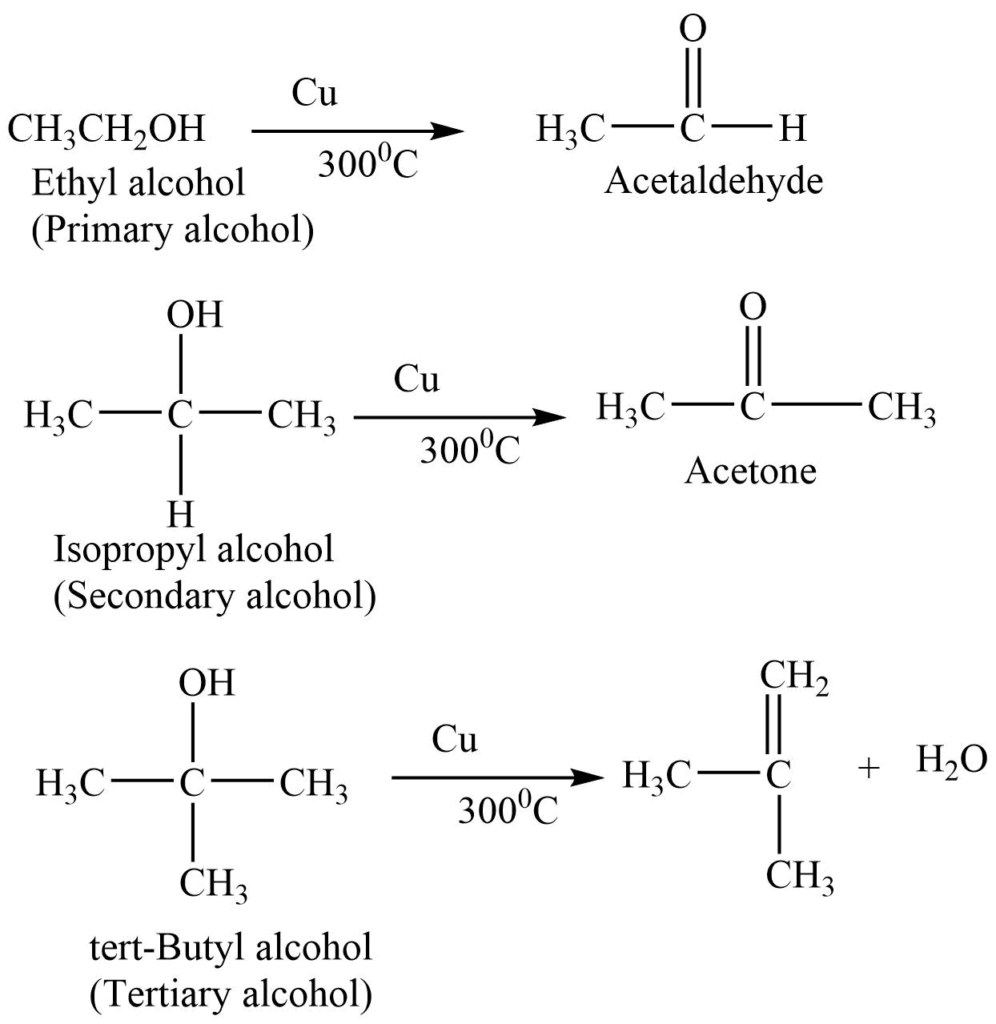
7. Dehydrogenation reaction
Alcohol vapor on passing over copper gauze at 3000C give different dehydrogenation products.
8. Lucas test
Alcohol on reaction with Lucas reagent ( ZnCl2 + HCl) gives alkyl chloride. different alcohols react with Lucas reagent at different rates. The resulting alkyl chloride is insoluble in the medium, causing the solution to appear cloudy. Tertiary alcohol reacts with Lucas reagent very rapidly, secondary alcohol reacts somewhat slowly while primary alcohol don,t react with Lucas reagent at room temperature. A high temperature is required for primary alcohol. This reaction is used to distinguish between primary, secondary, and tertiary alcohol.
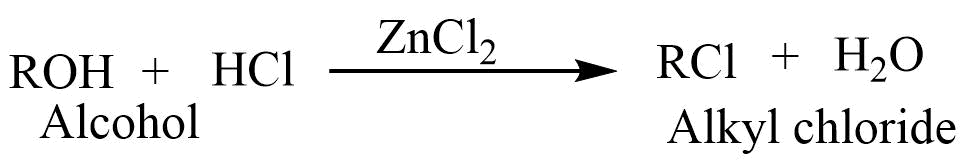
Uses of Alcohols
- Alcohol can be used as a solvent.
- Alcohols are used as starting materials for organic synthesis reactions.
- Methanol and Ethanol can be used as fuel.
- They are used to make spirits.
- Ethanol is also used for the preparation of alcoholic drinks.
- Alcohols are used as antibacterial, antifungal, antiseptics, and disinfectants in medicine in various forms.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- https://www.britannica.com/science/organic-compound
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols
- https://byjus.com/chemistry/types-of-alcohols/
- https://www.chemistrysteps.com/oxymercuration-demercuration/
- https://byjus.com/chemistry/lucas-test/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols/Properties_of_Alcohols/Uses
- https://www.chemguide.co.uk/organicprops/alcohols/uses.html
- https://byjus.com/chemistry/uses-of-methanol-and-ethanol/
